Tlr7/8 antagonists and uses thereof
A technology of -CO2R and -NRSO2R, applied in the field of TLR7/8 antagonist and its application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0444] Example 1. Pharmaceutical preparations
[0445] (A) Injection vial: a solution of 100 g of the active ingredient of the present invention and 5 g of disodium hydrogen phosphate in 3 liters of double distilled water was adjusted to pH 6.5 using 2N hydrochloric acid, sterile filtered, transferred to an injection vial, and frozen under aseptic conditions. Dry and seal under sterile conditions. Each injection vial contains 5 mg of active ingredient.
[0446] (B) Suppositories: 20 g of the active ingredient of the invention are melted with a mixture of 100 g of soy lecithin and 1400 g of cocoa butter, poured into molds and allowed to cool. Each suppository contains 20 mg of active ingredient.
[0447] (C) solution: in 940ml heavy distilled water, by 1g active ingredient of the present invention, 9.38gNaH 2 PO 4 2H 2 O, 28.48gNa 2 HPO 4 12H 2 O and 0.1 g benzalkonium chloride to prepare a solution. The pH was adjusted to 6.8 and the solution was made up to 1 l and ster...
Embodiment 2
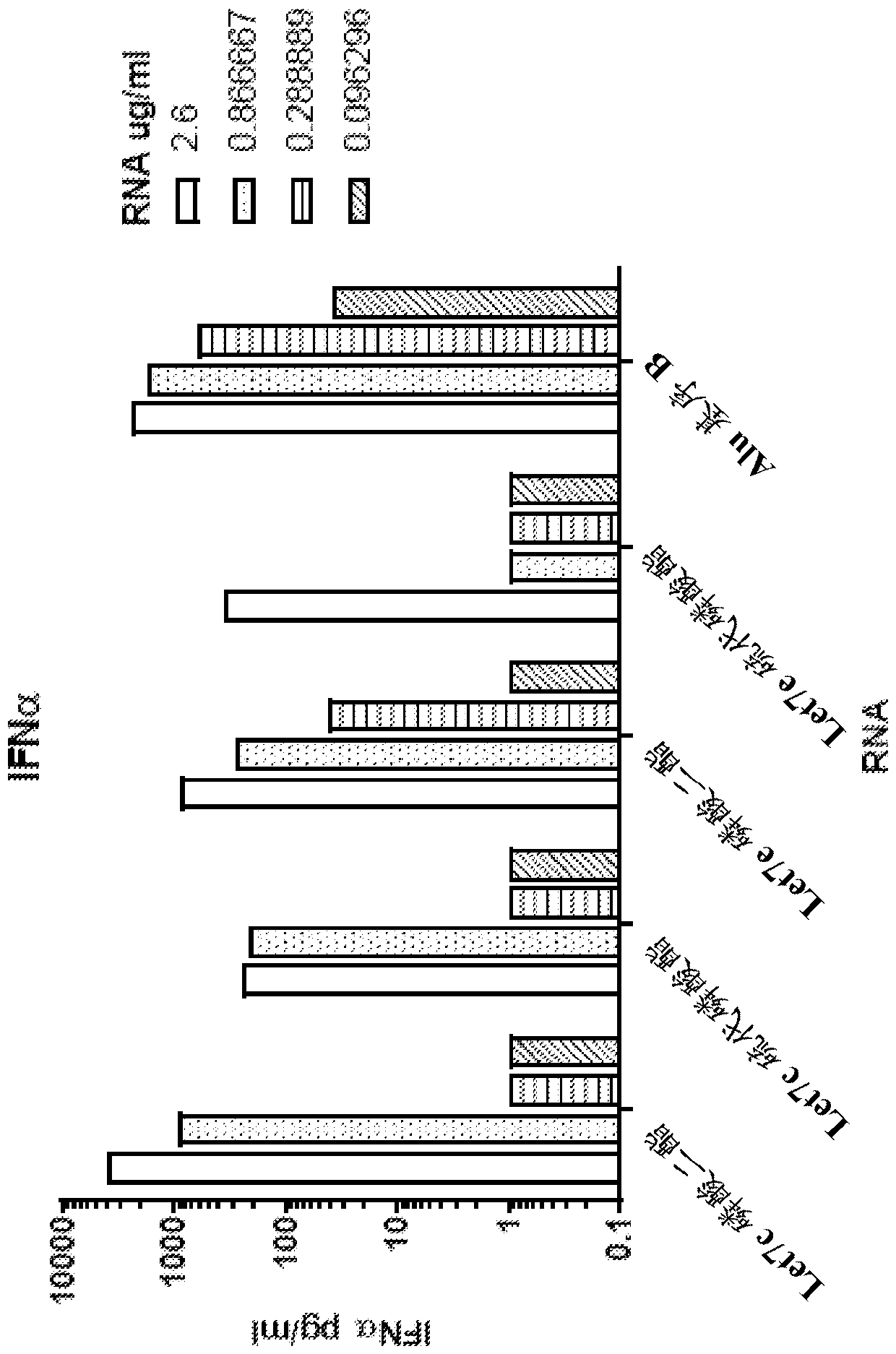
[0463] Human peripheral blood lymphocytes were transfected with let-7c and let-7e miRNAs. 24 hours after transfection, IL-6 in the cell supernatant was analyzed ( figure 1 ) and IFNα ( figure 2 ) presence of cytokines. Two forms of RNA oligonucleotides with phosphate or phosphorothioate linkages were used, respectively.
[0464] Hu let-7c UGAGGUAGUAGGUUGUAUGGUU (+ / - phosphorothioate bond)
[0465] Hu let-7e UGAGGUAGGAGGUUGUAUAGUU (+ / - phosphorothioate bond)
[0466] Alu motif B
[0467] UUUUUUUUUUUUUUUUUUUUUUUUUGAGACGGAGUCUCGCUCUGUCGCC (only diester bonds)
[0468] These findings demonstrate that delivery of let7 miRNA to human PBMCs induces IFNα and IL-6 production in a dose-dependent manner.
Embodiment 3
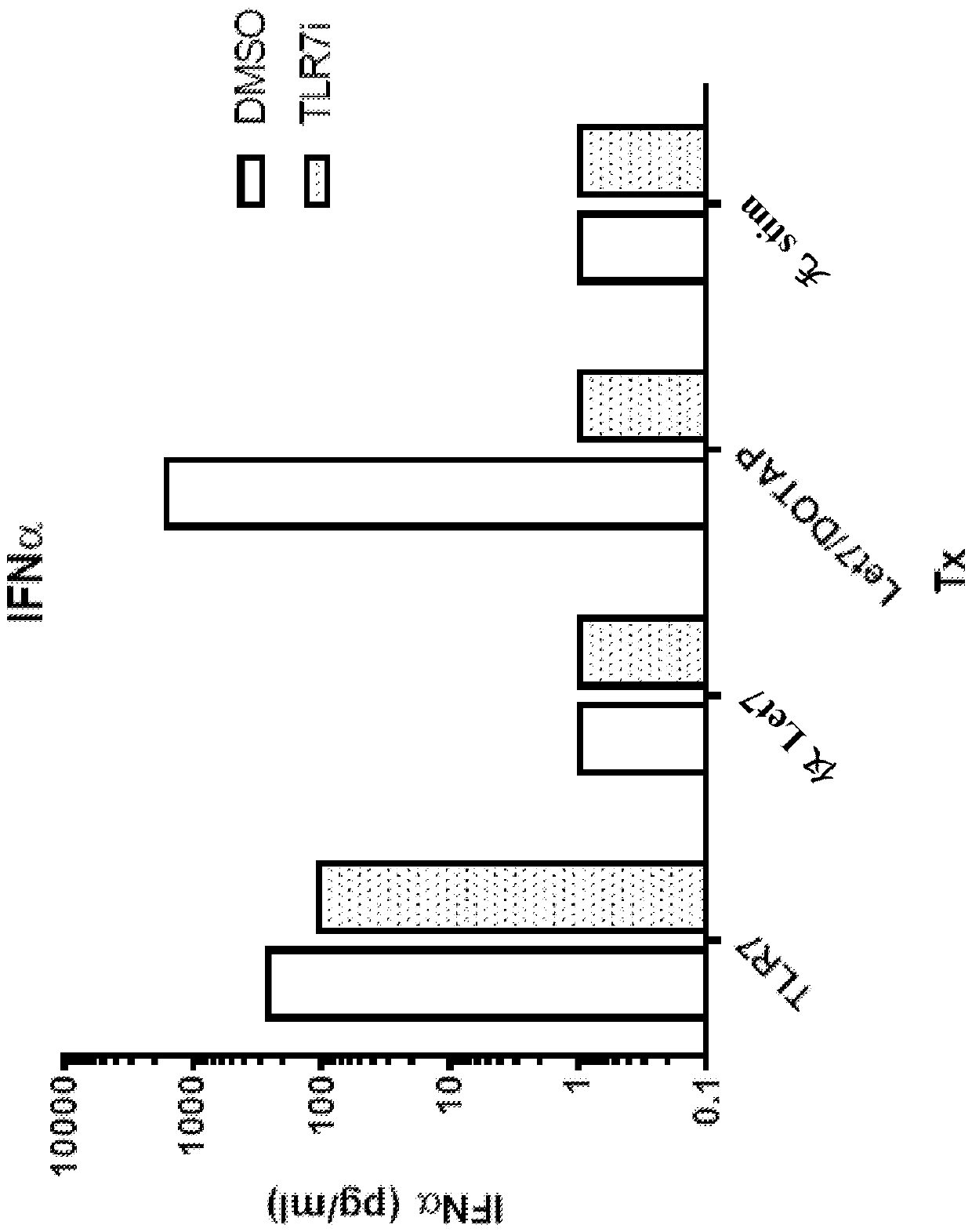
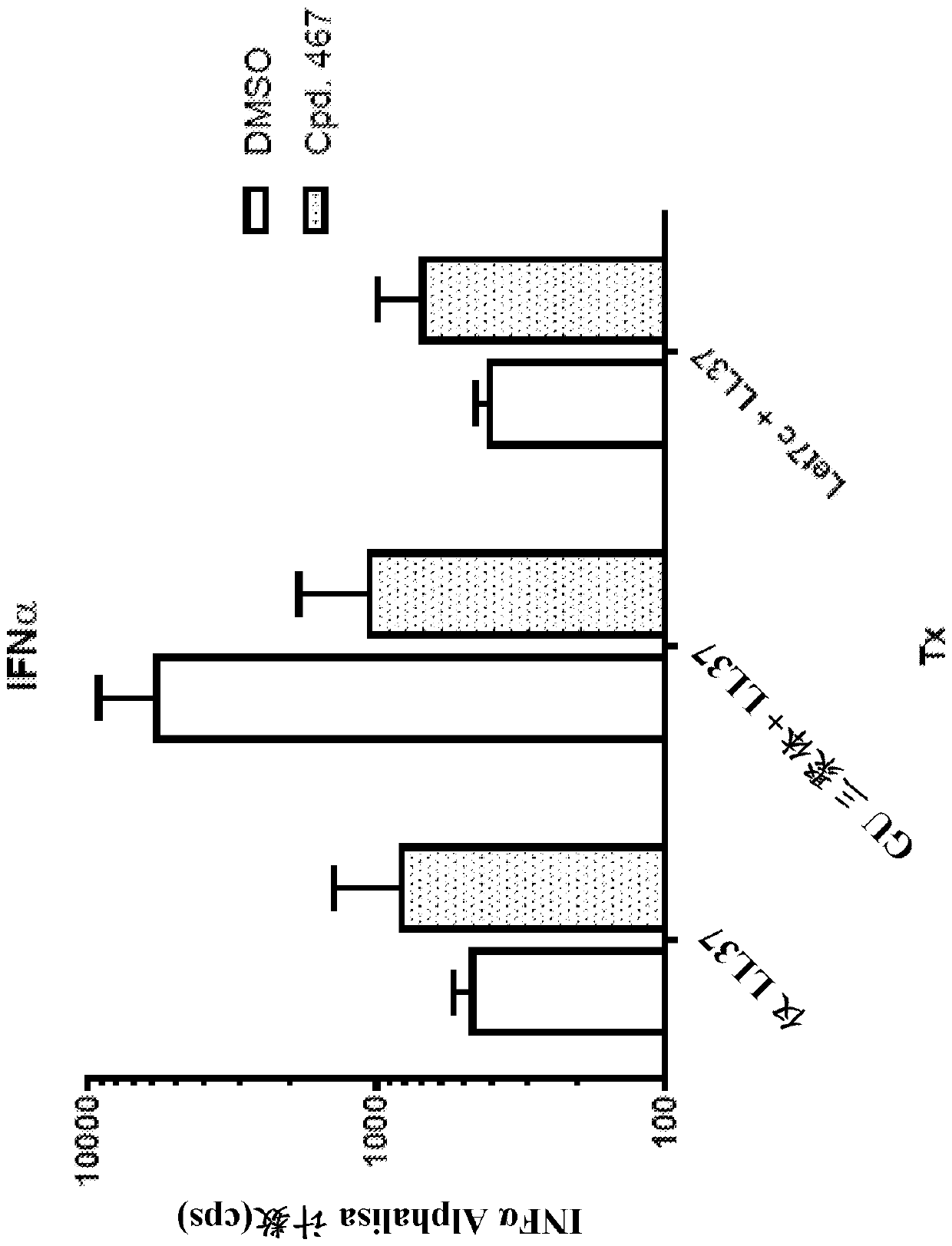
[0470] Human PBMC were treated with a TLR7 agonist (TLR7), let7c miRNA or transfected with let7c miRNA (let7 / DOTAP) in the presence of a TLR7 / 8 antagonist (compound 467 in Table 1 above). After overnight incubation, IL-6 ( image 3 ) and IFNα ( Figure 4 )s level.
[0471] These findings demonstrate that small-molecule TLR7 / 8 antagonists block let-7 miRNA-induced cytokine production in human PBMCs.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com