Application of plasma-free replacement liquid composition in preparation of medicine for removing M protein of multiple myeloma
A technology of multiple myeloma and plasma exchange, applied in the field of medicine, can solve the problems of kidney damage, high patient expenses, and limited clinical application effects, so as to improve hyperviscosity syndrome, avoid multiple complications, and reduce the risk of transmission Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
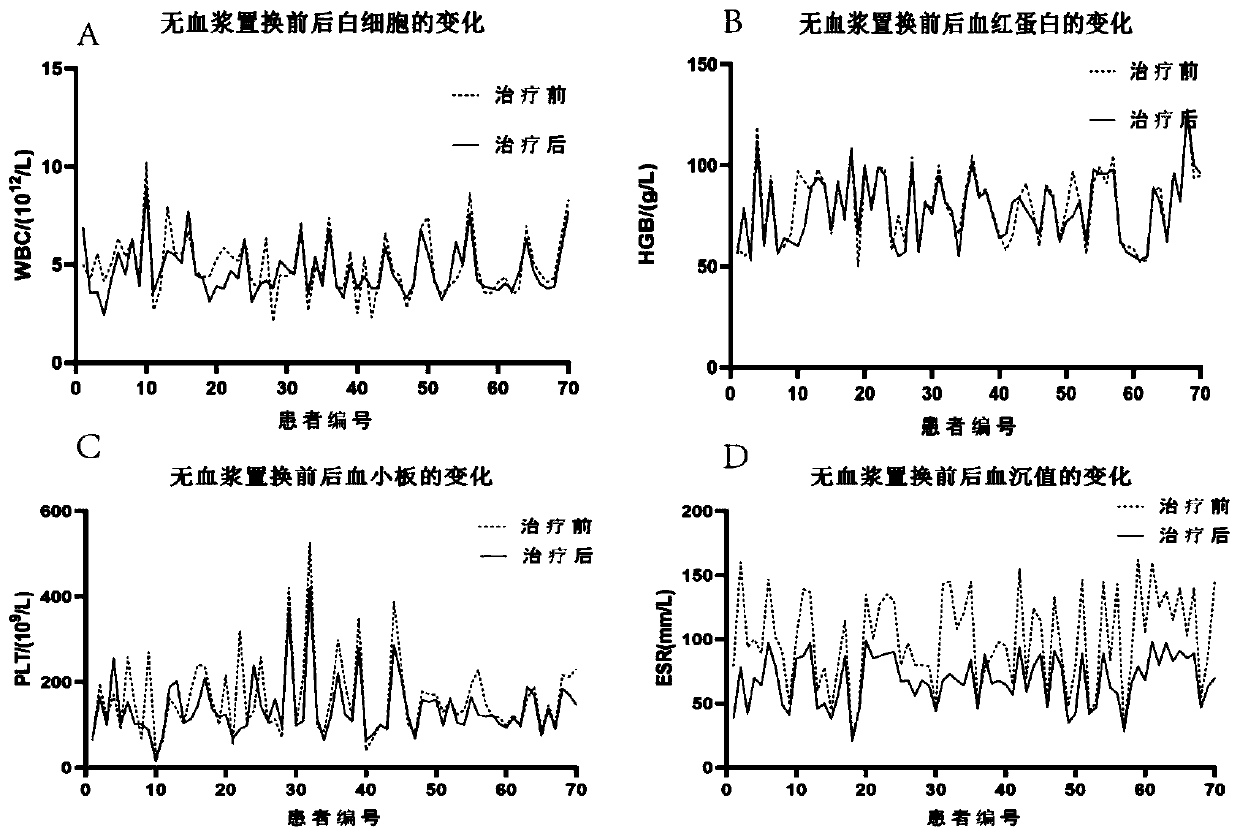
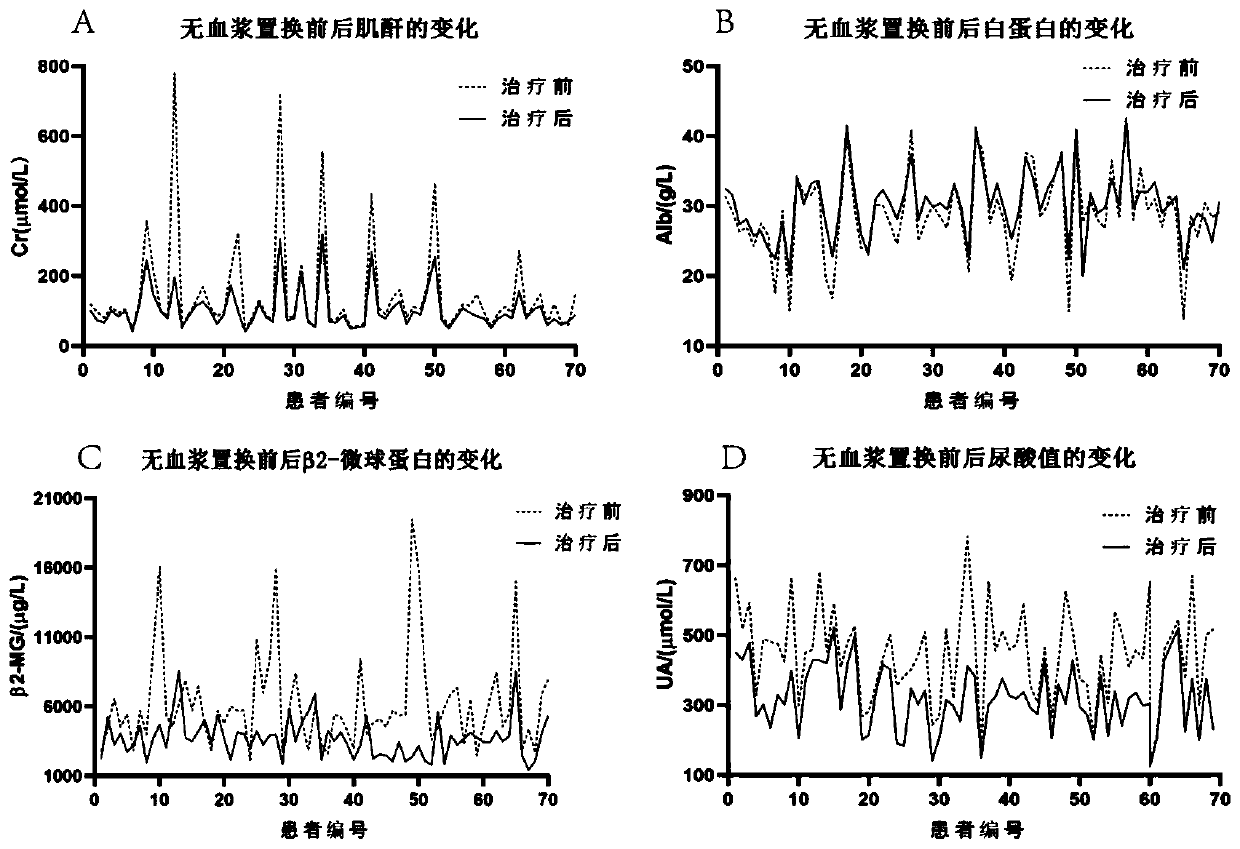
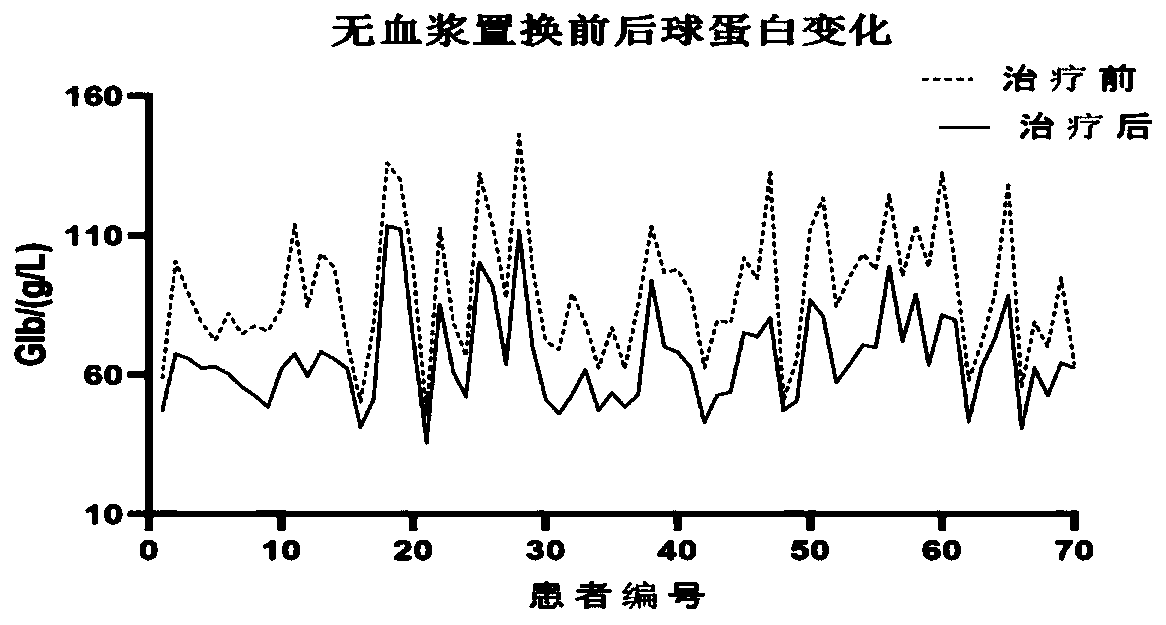
[0028] The features and advantages of the present invention can be further understood through the following detailed description in conjunction with the accompanying drawings. The examples provided are only illustrative of the method of the present invention and do not limit the rest of the present disclosure in any way. [Example 1] 70 cases of newly diagnosed multiple myeloma patients were treated with plasma exchange using albumin-free plasma exchange composition
[0029] 1. Materials and methods
[0030] 1.1. General Information
[0031] A total of 70 patients with MM admitted to our hospital from February 2016 to August 2018 were selected as the research subjects, all of whom were newly diagnosed cases and had not undergone other forms of plasma exchange therapy. Among them, there were 40 males and 30 females; the age ranged from 48 to 71 years, with an average age of (59.56±5.47) years; the course of disease was 1 to 3 years, with an average duration of (1.55±0.31) year...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com