Broad-spectrum organophosphorus pesticide aptamer as well as method and application thereof
An organophosphorus pesticide and aptamer technology, applied in biochemical equipment and methods, material testing products, instruments, etc., can solve the problems of wide detection range and low detection sensitivity, and achieve the effect of low synthesis cost and improved affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
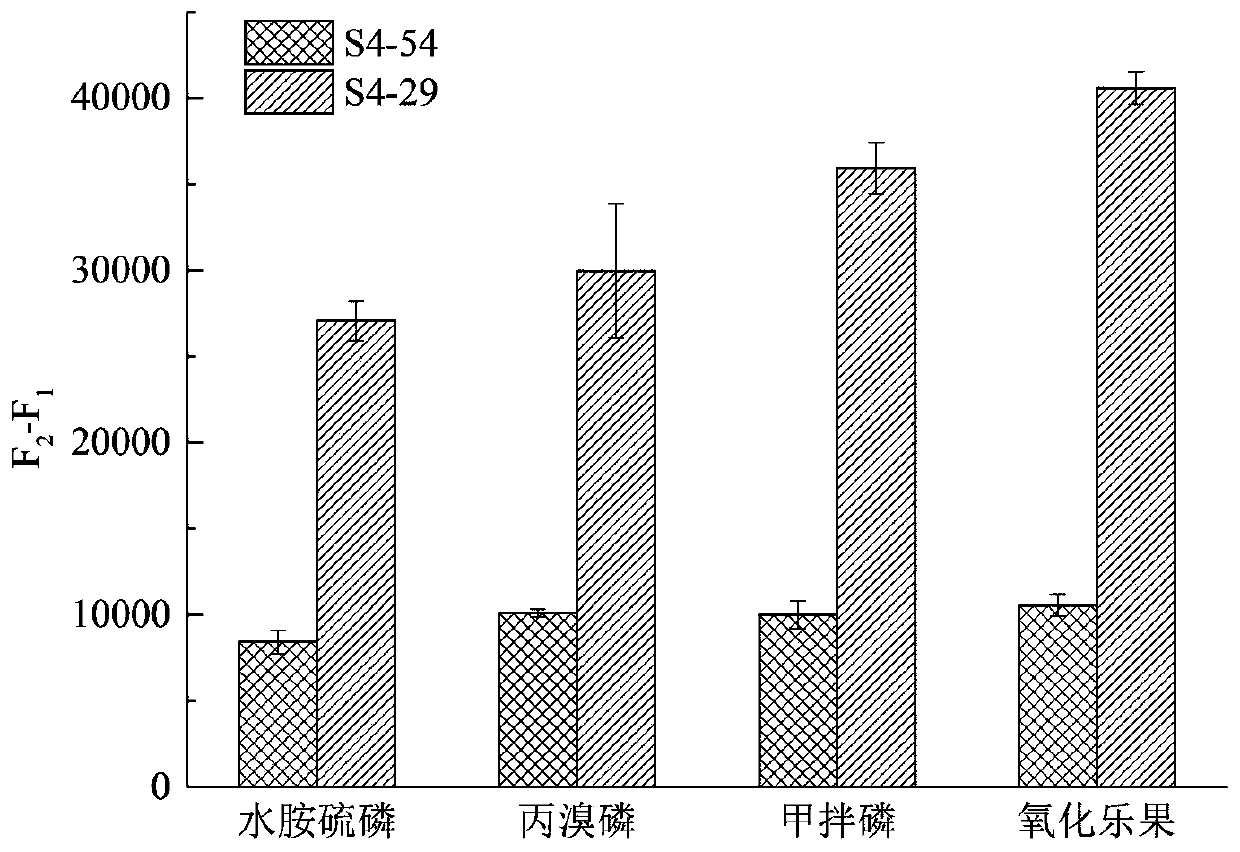
[0034] A broad-spectrum organophosphorus pesticide aptamer, the nucleotide sequence of the aptamer is the sequence shown in SEQ.ID NO7 (S4-29), and the sequence is shown in Table 1 below.
experiment example 1
[0041] Binding free energy of experiment example 1 aptamer
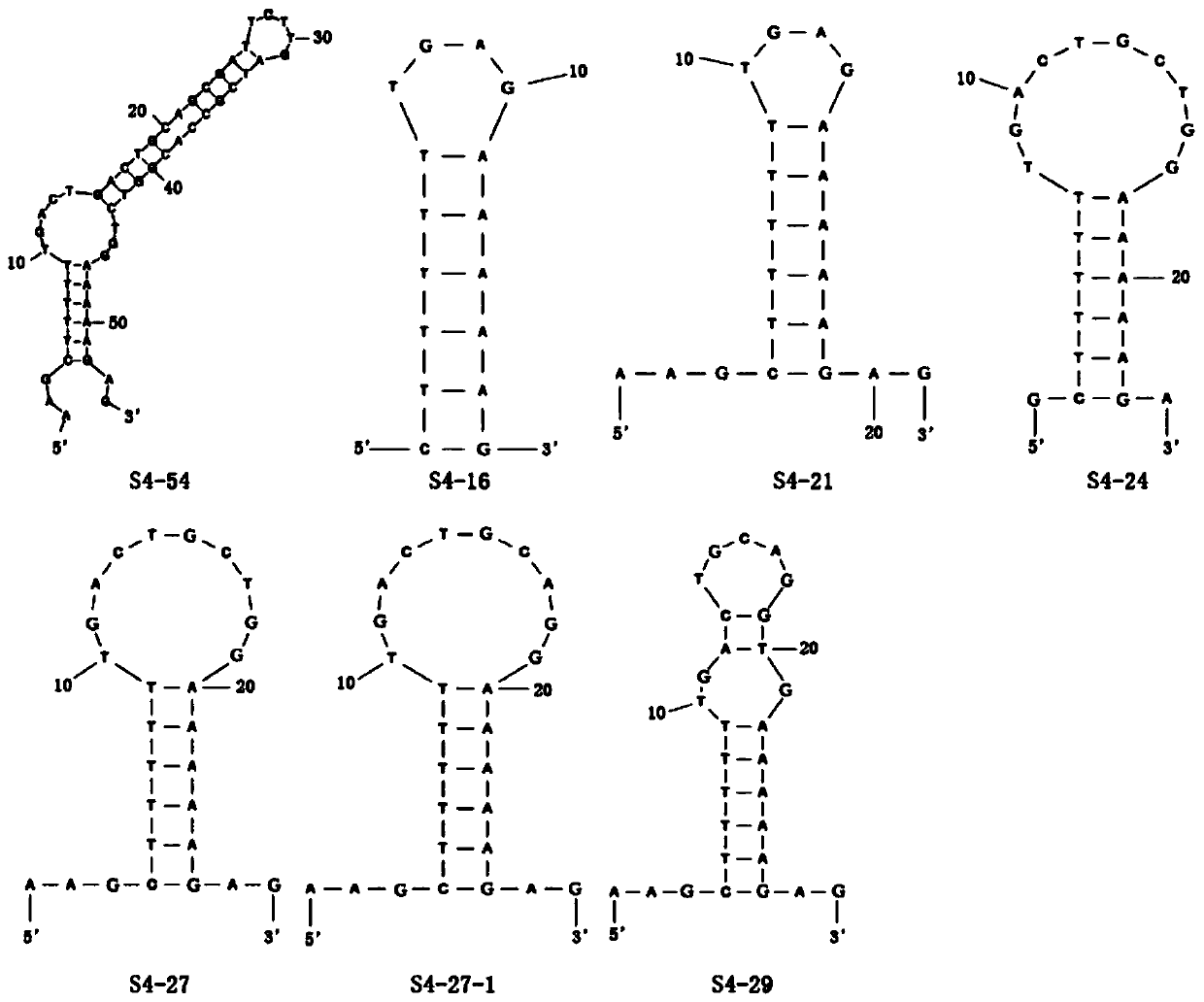
[0042] Such as figure 1 As shown, it is the secondary structure diagram of the nucleotide sequence of the aptamer of Example 1 and S4-54 of the present invention and Comparative Example 1. The 3D structure of the aptamer model is obtained through the secondary structure, and the RNA and DNA are completed. Structural transformations. Optimize the structure of pesticides (isocarbophos, omethoate, methamidophos and profenofos), and dock the optimized ligand (pesticide structure) with the aptamer to obtain the three-dimensional structure of the complex ligand-ligand structure.
[0043]The binding free energy was obtained after molecular docking, as shown in Table 2. Taking the existing aptamers S4-54 and S24 as comparisons, it can be seen that the aptamers S4-16, S4-21 and S4-24 in Comparative Example 1 The binding ability of aptamer S4-54 is worse than that of the existing aptamer S4-54, which is higher than that of S...
experiment example 2
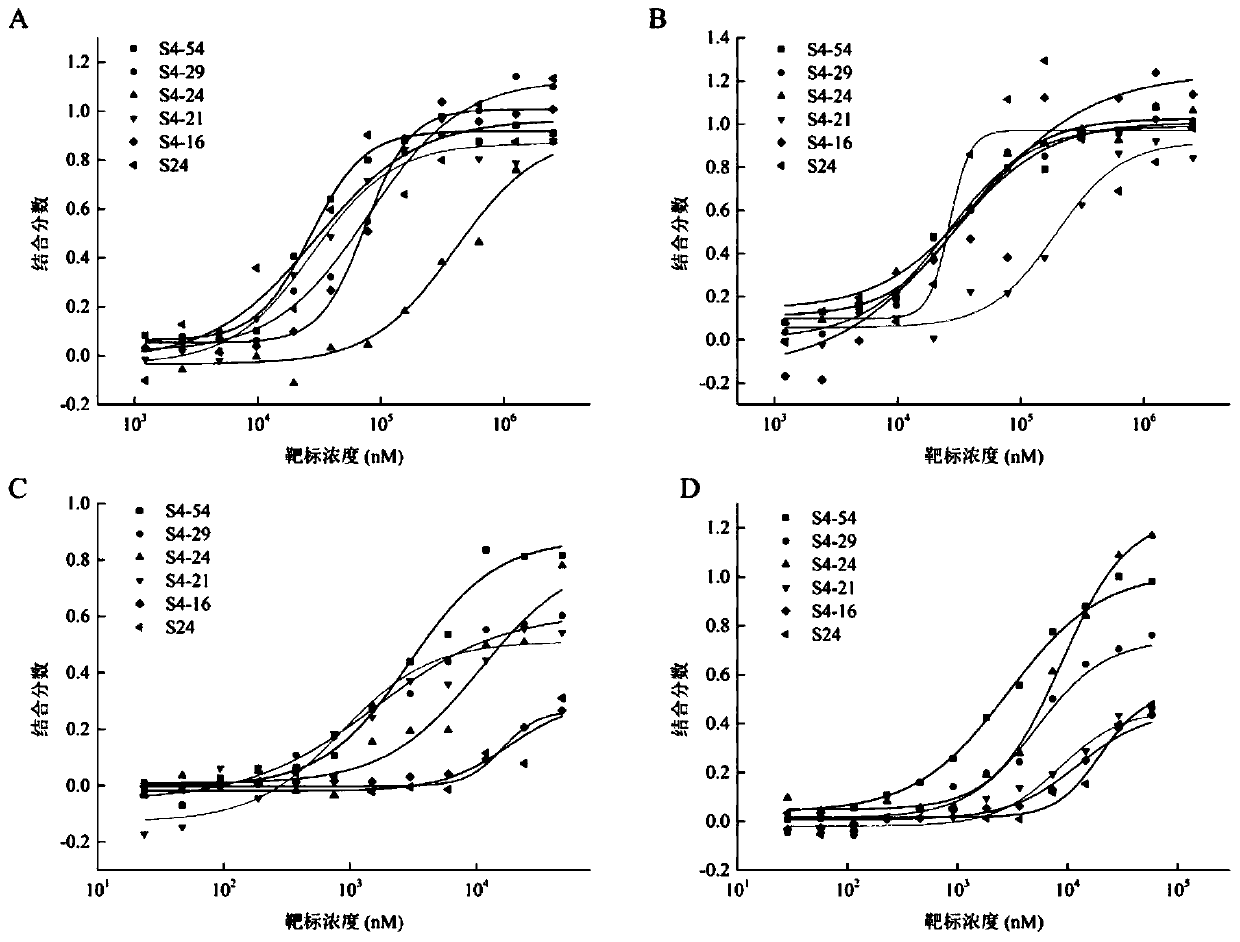
[0046] Experimental Example 2 Affinity analysis of aptamers
[0047] The affinity of the aptamers S4-16, S4-21, S4-24 and S4-29 of the present invention and the existing aptamer S4-54 was analyzed, and the aptamers were labeled with the fluorescent group FAM at the 5' end, specifically as follows:
[0048] First, with binding buffer (50mmol / L Tris, 50mmol / L NaCl, 10mmol / L KCl and 10mmol / L MgCl 2 , pH 8.0) dilute the aptamer to a certain concentration, denature at 90°C for 5min, and immediately ice-bath. Next, use the binding buffer to prepare a high-concentration pesticide solution, the pesticide is isocarbophos, profenofos, phorate or omethoate, use the high-concentration pesticide solution as the highest concentration of the ligand, and make a sample volume of 10 μL 16 samples were prepared using a 1:1 serial dilution strategy in . Then, 10 μL of aptamer solution was added to each sample. All samples were reacted at room temperature in the dark for 30 min, and loaded to ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap