Patents
Literature
62results about How to "Short sequence" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
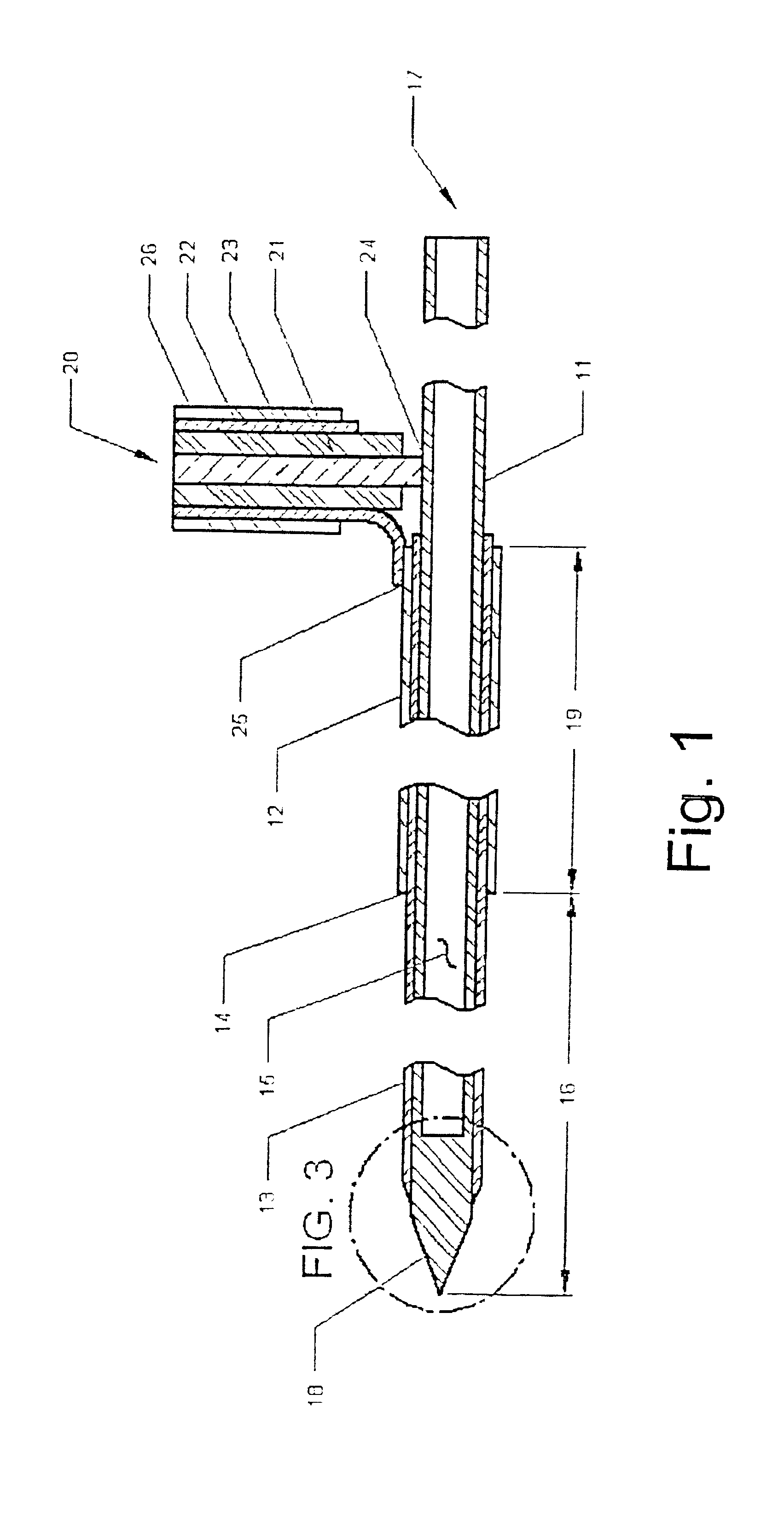
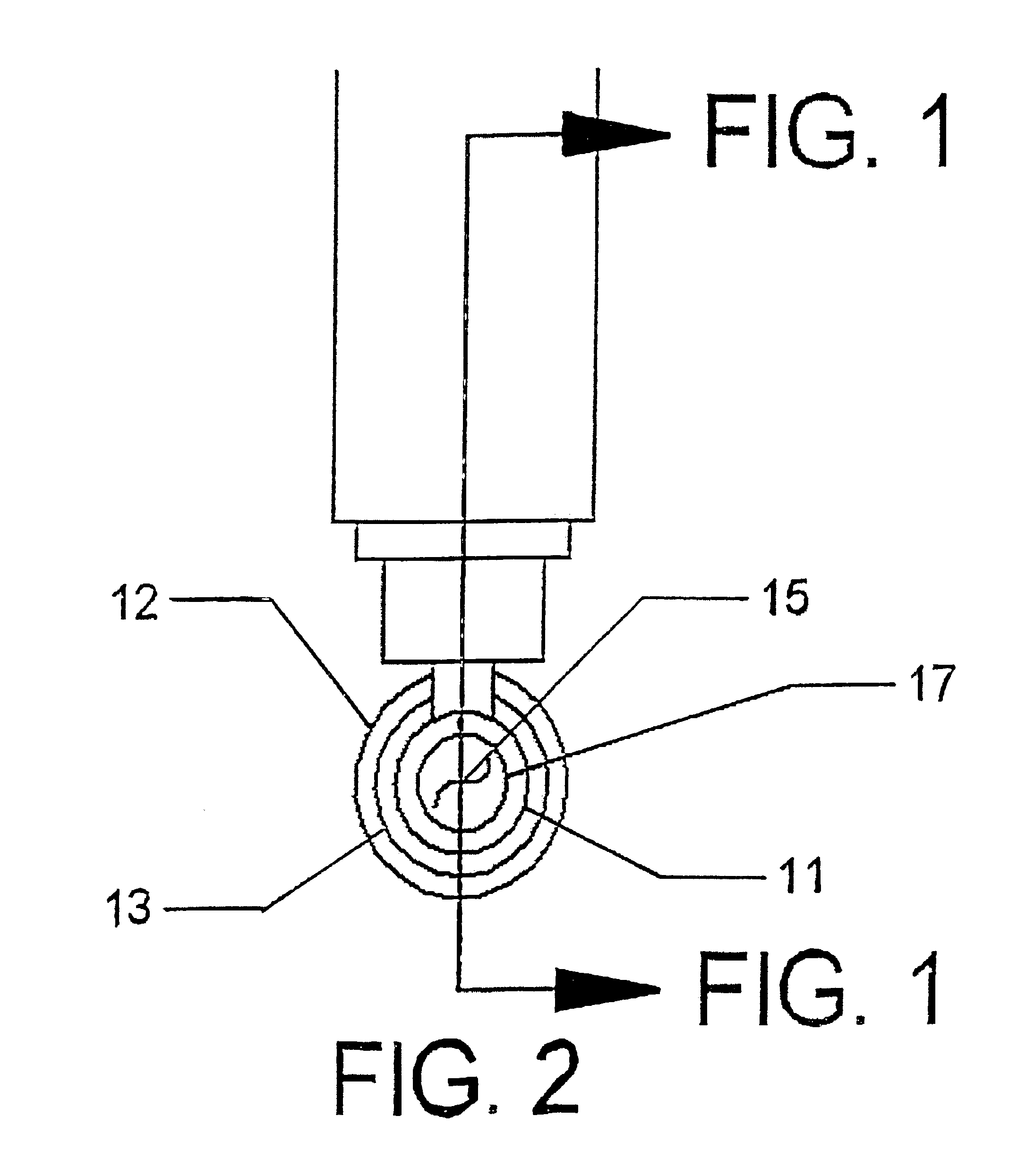
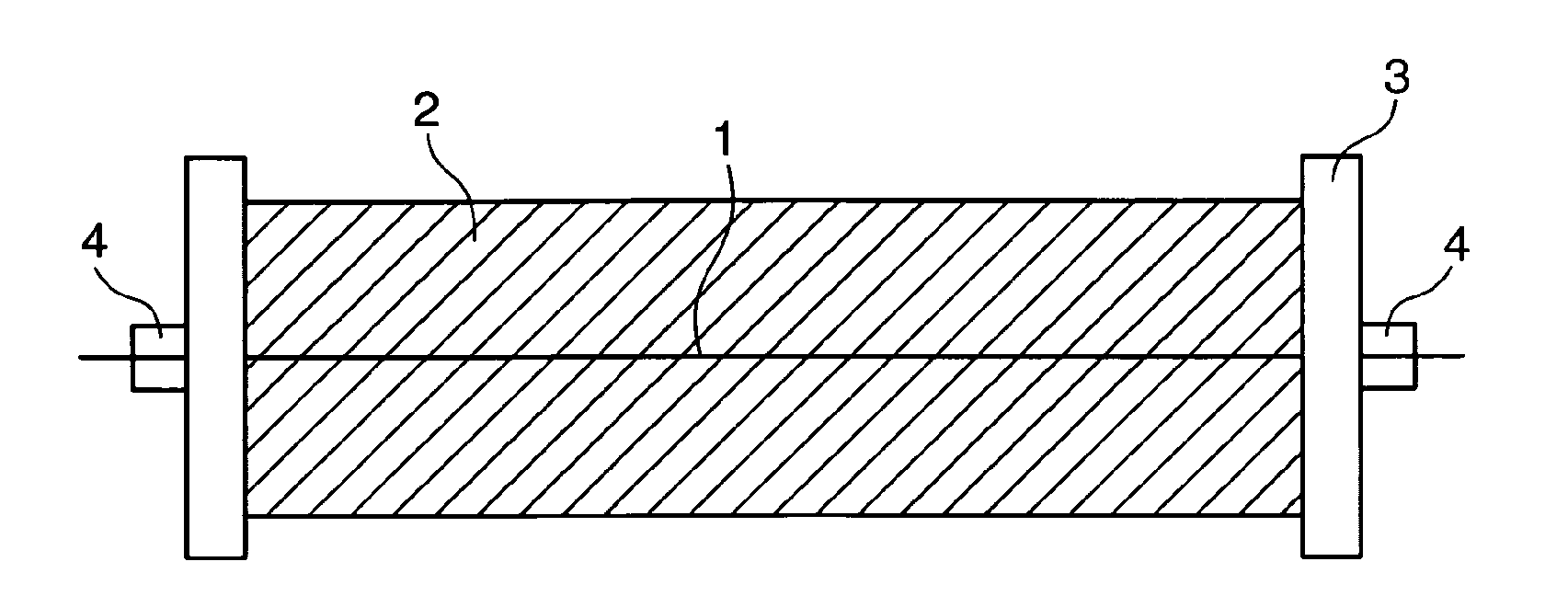
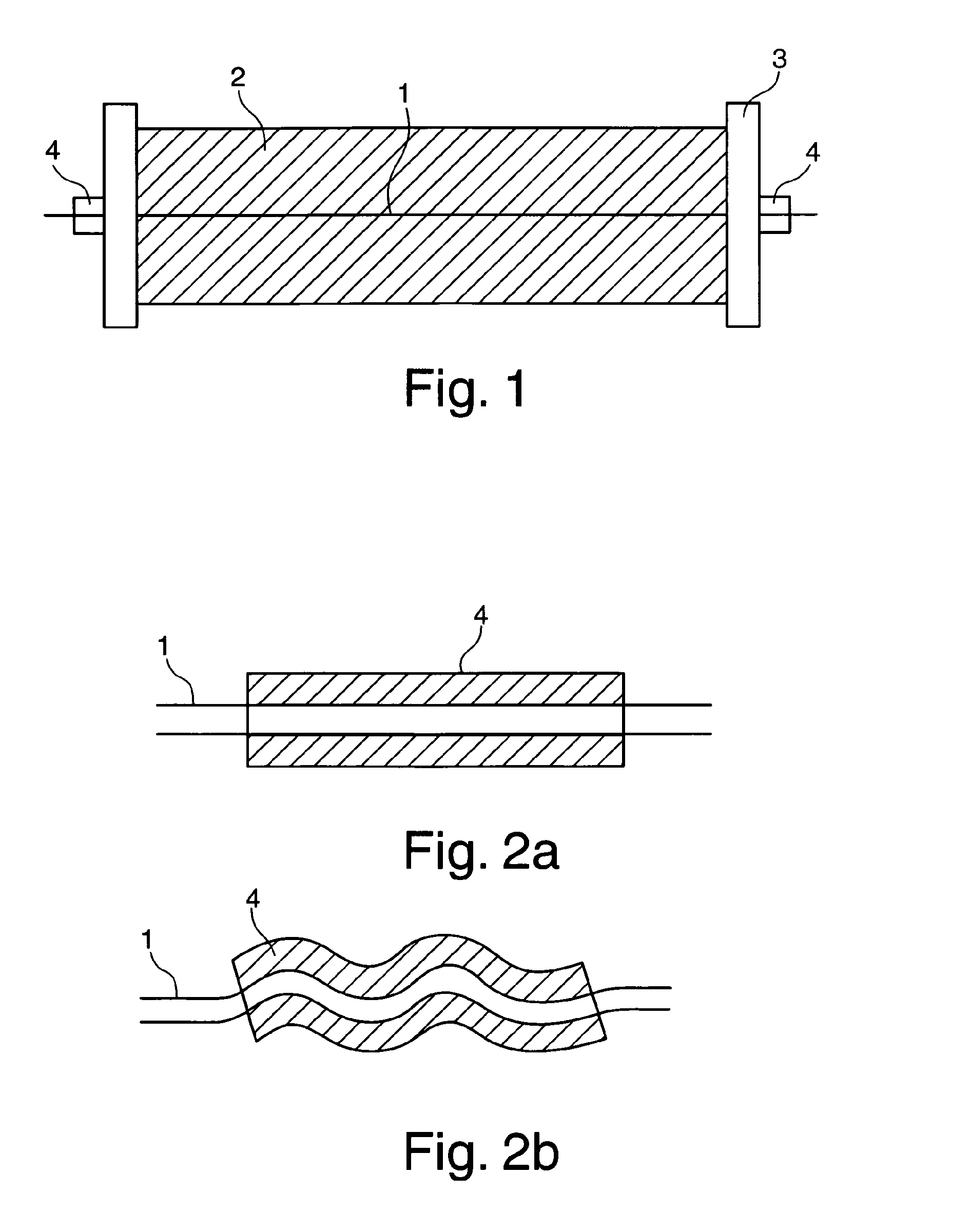
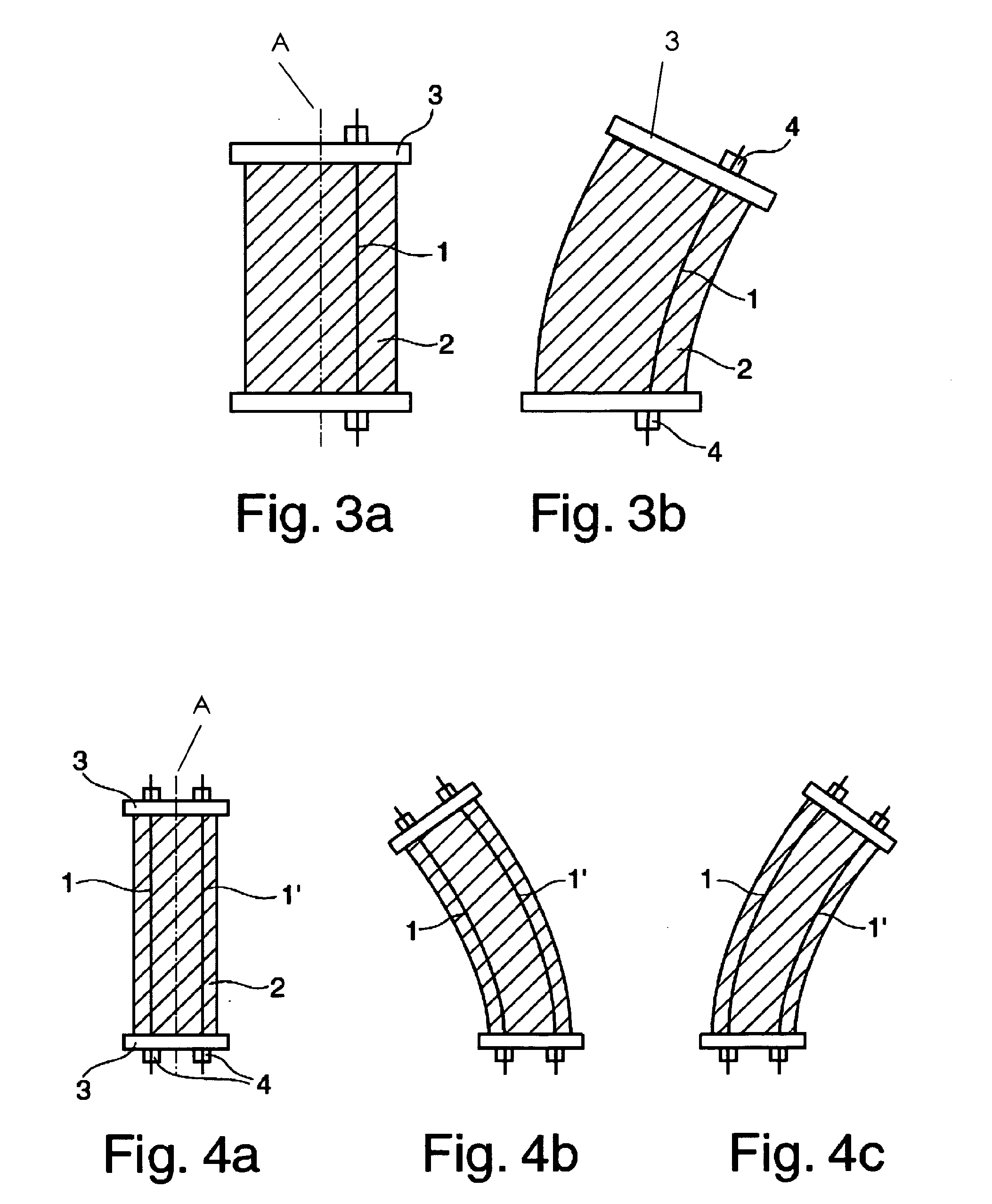
Invasive microwave antenna array for hyperthermia and brachytherapy
ActiveUS20040243200A1Increase oxygenationEnhance tumor blood flowMicrowave therapySurgical instruments using microwavesElectrical conductorEngineering
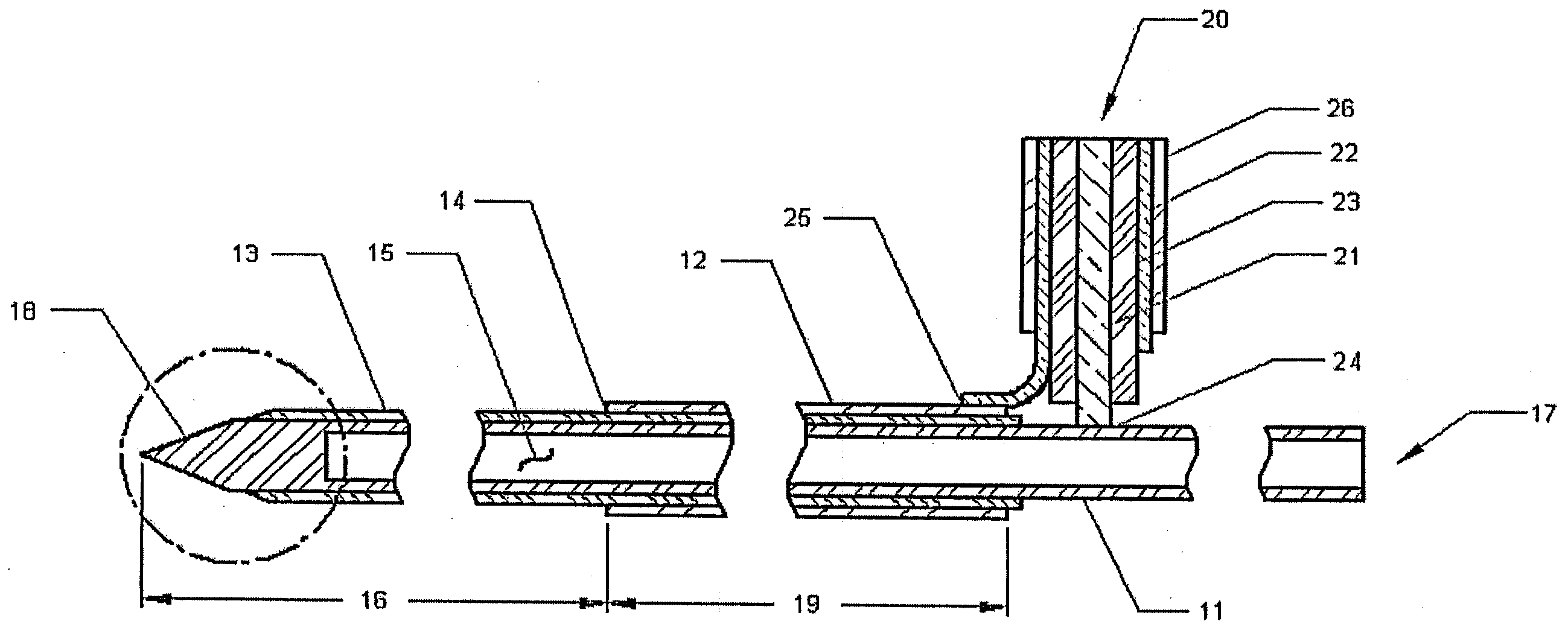
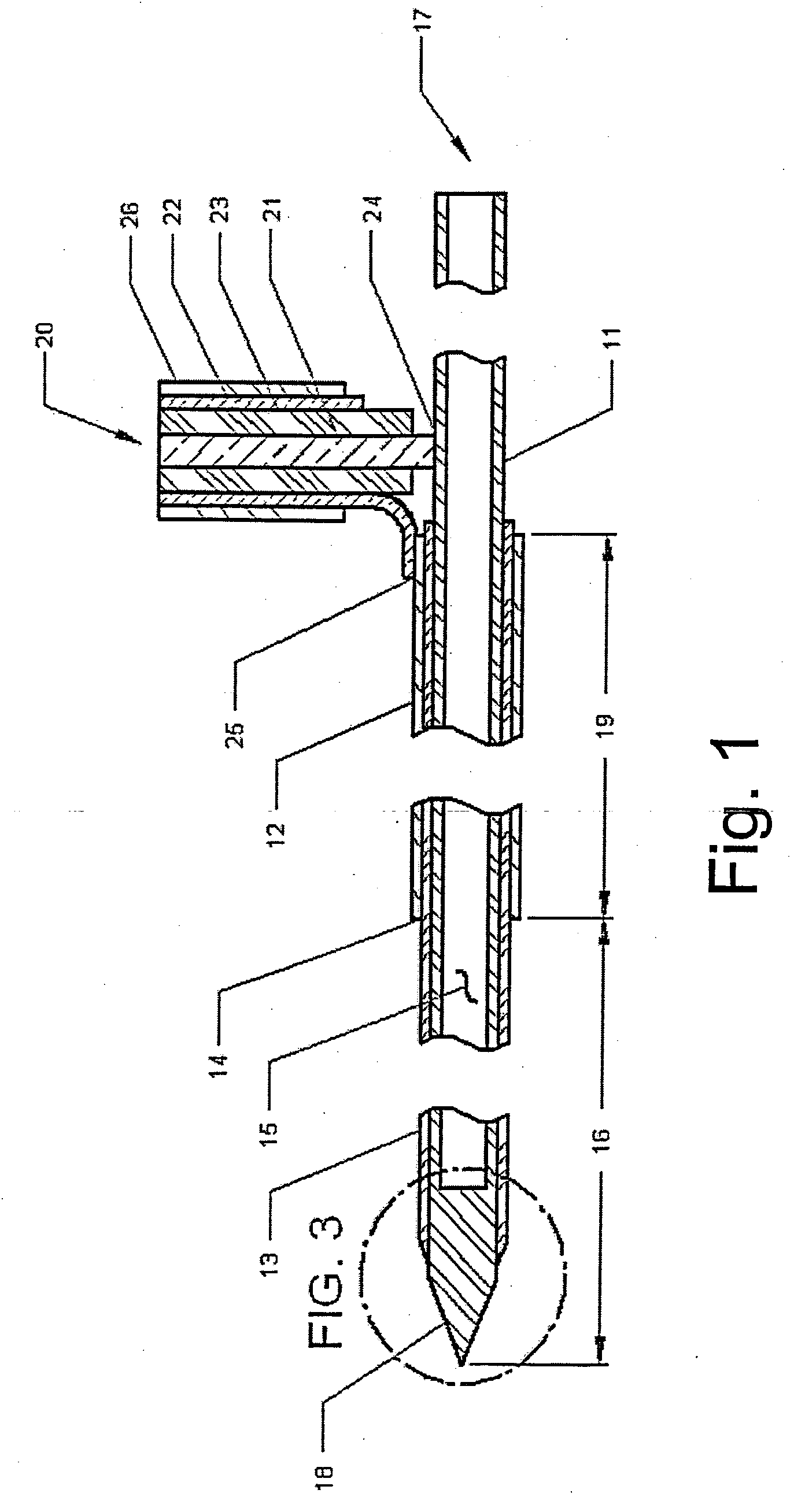
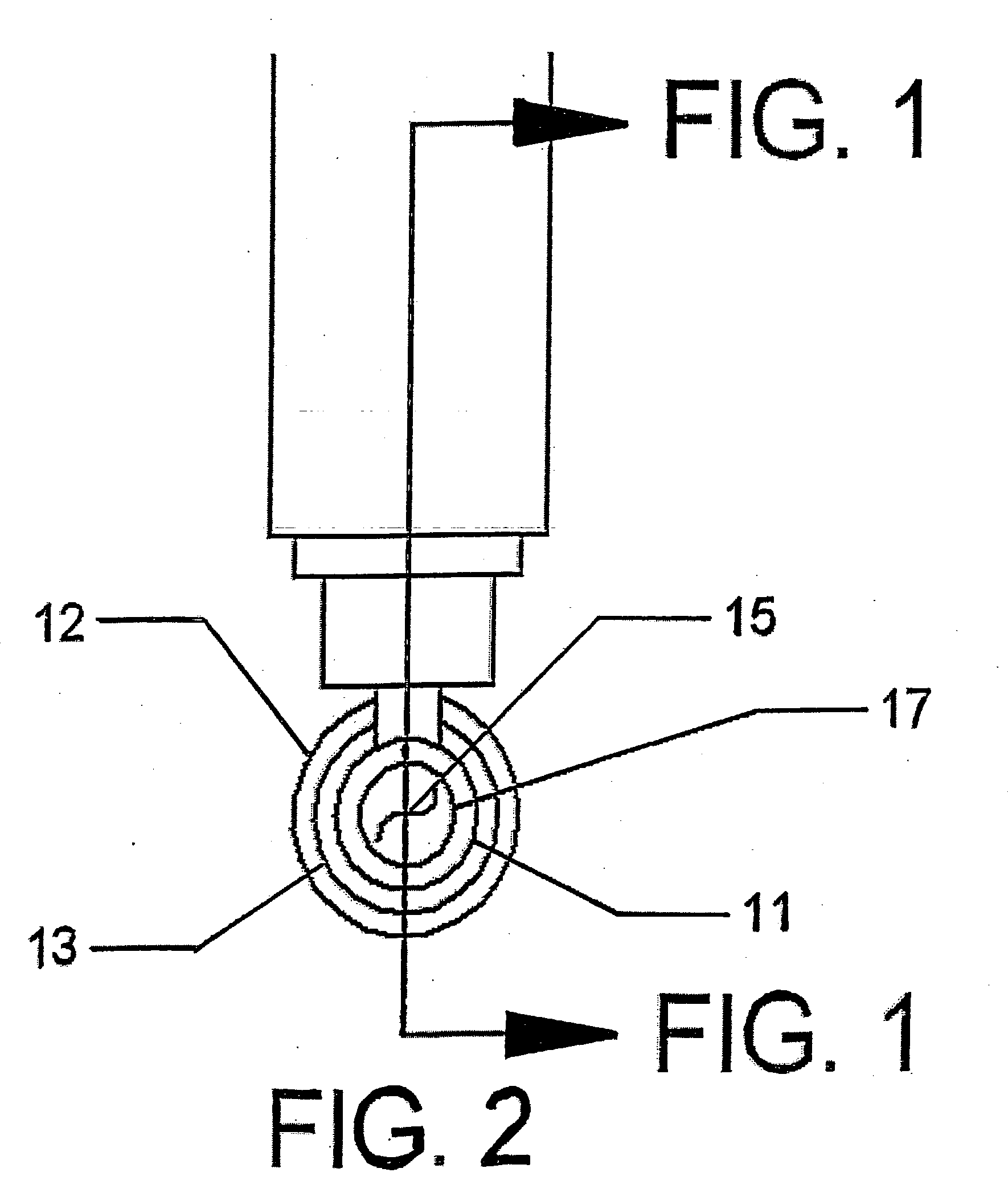
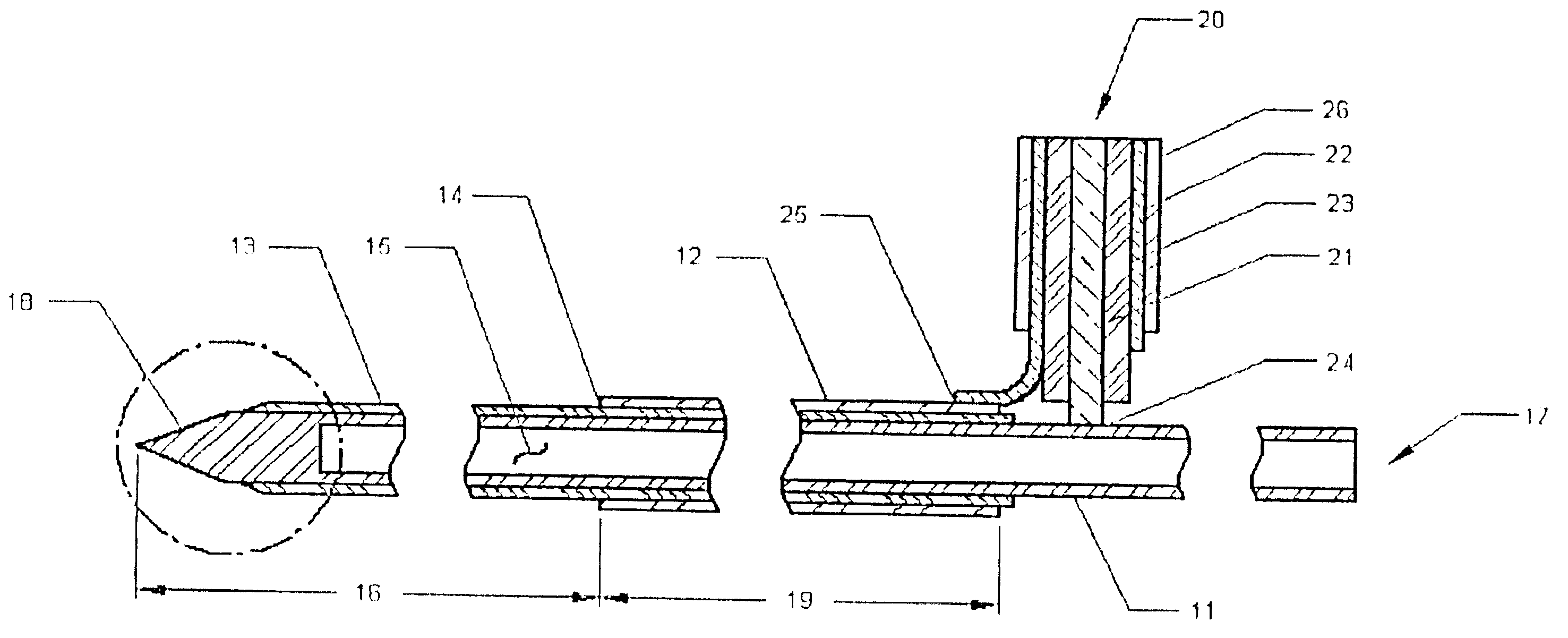
A microwave hyperthermia apparatus that can be inserted into the body which includes a hollow central tube for the insertion of radioactive therapy sources. The use of a coaxial transmission line impedance transformation along the insertable portion of the coaxial cable enables a reduction in the characteristic impedance by increasing the outer diameter of the inner coaxial conductor so that the center conductor can be a metal tube. If the ratio of the outer coaxial conductor diameter vs. the inner coaxial conductor is decreased, the characteristic impedance of the transmission line is lowered. This enables the inner conductor diameter to increase sufficiently to make the central hollow opening large enough to receive standard radioactive sources therein. This provides for a good impedance match that improves microwave energy efficiency while at the same time permitting a large hollow center opening. The combination of the microwave antenna device and brachytherapy sources provides for enhanced effectiveness when the two treatments are delivered simultaneously or in close time proximity to each other.
Owner:PYREXAR MEDICAL
Invasive microwave antenna array for hyperthermia and brachytherapy
InactiveUS6957108B2Increase oxygenationSpeed up the flowMicrowave therapySurgical instruments using microwavesElectrical conductorEngineering
Owner:PYREXAR MEDICAL
System and method using alphanumeric codes for the identification, description, classification and encoding of information
InactiveUS20070162412A1Easy accessFast and accurate and contextually relevantSpecial data processing applicationsWeb data browsing optimisationComputer scienceGreek letter alpha
A method of accessing or receiving information stored in electronic or other form, the method comprising the use of alpha-numeric sub-codes, wherein the codes comprise one or more alpha-numeric sub-codes in a hierarchical structure and wherein the sub-codes and / or codes are used to identify, describe, define, classify or encode a description of the content of said information.
Owner:PERCY RICHARD
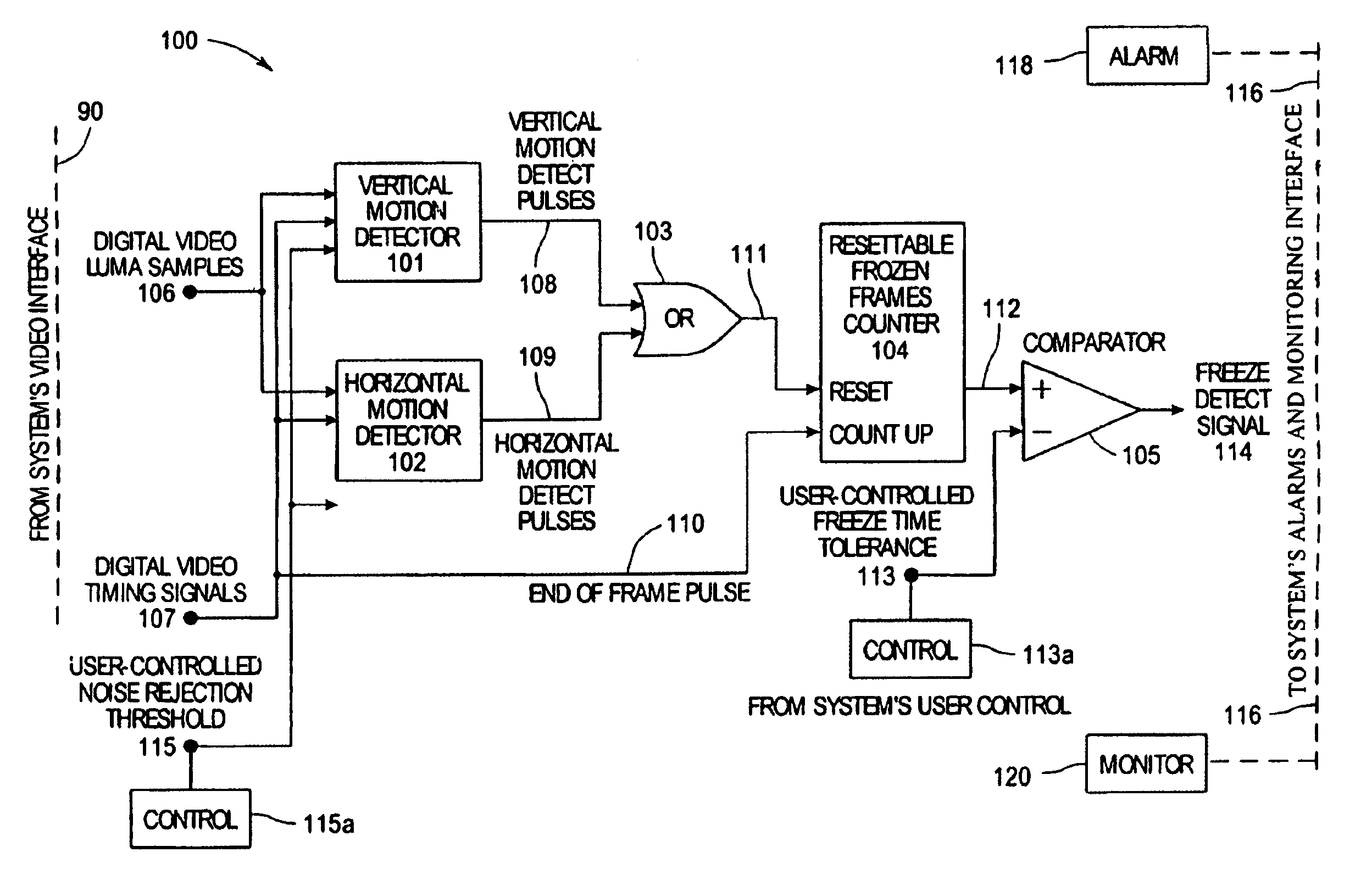
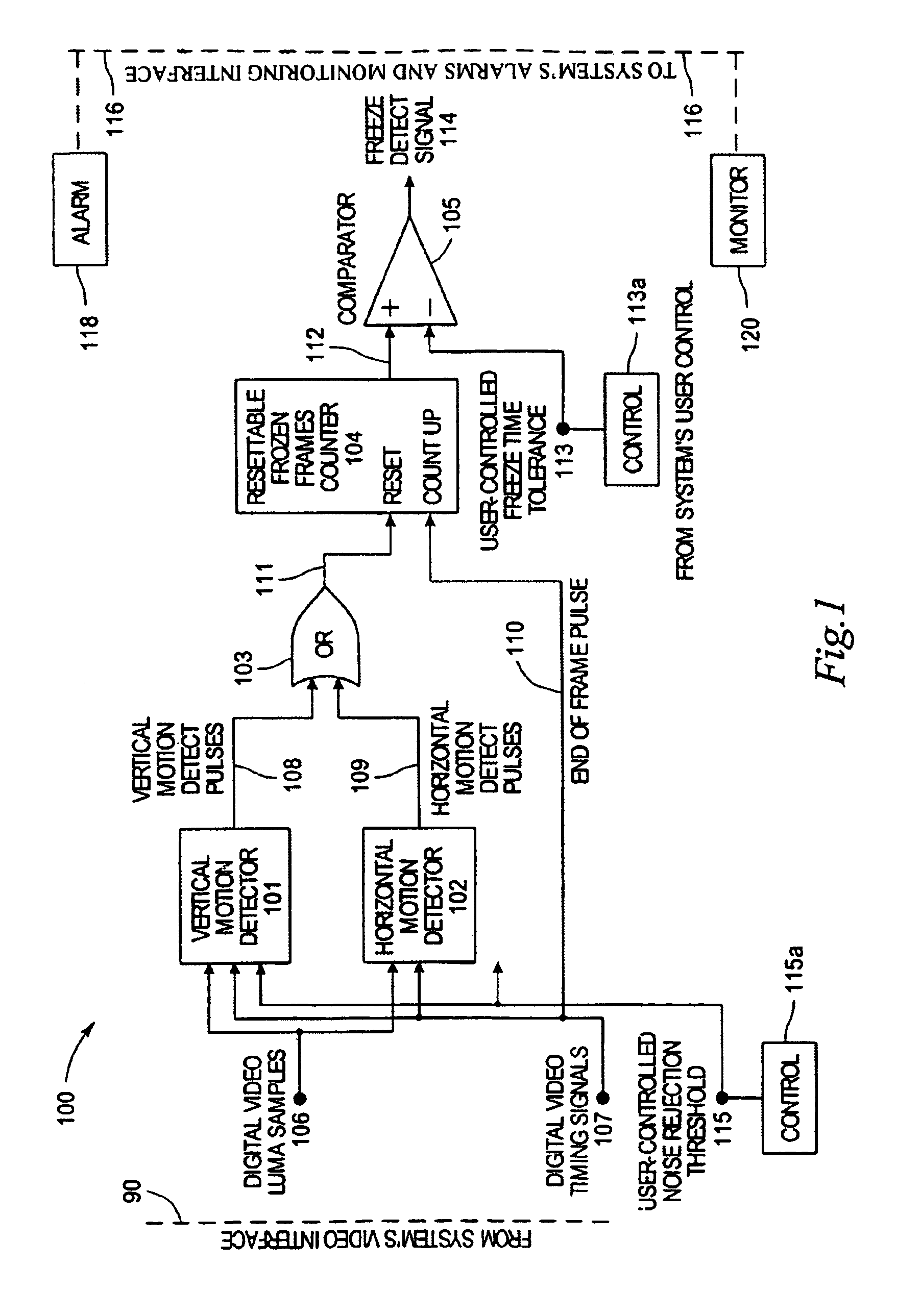
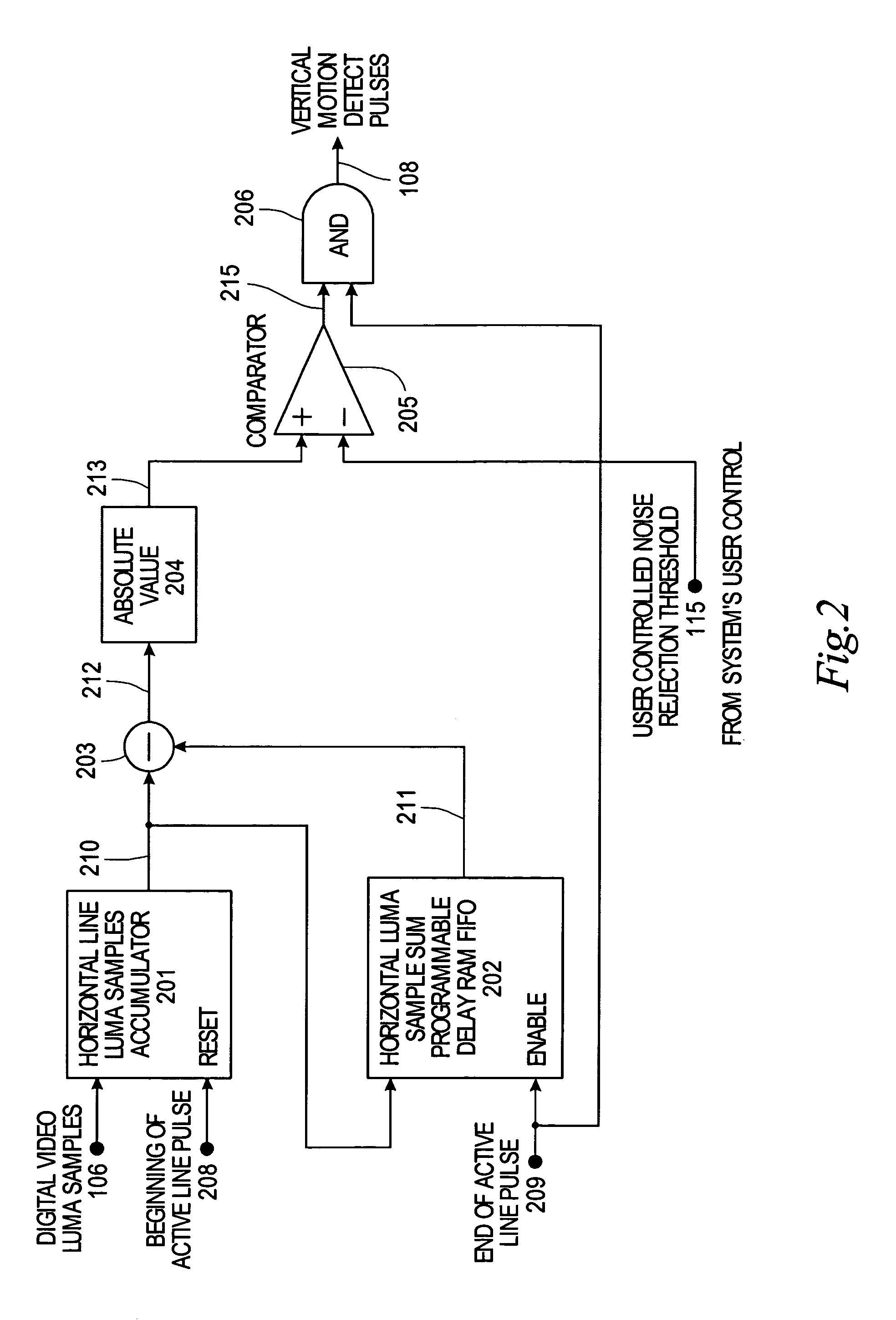
System and method for detecting picture freeze within a video signal
InactiveUS7002637B2Short sequenceMinimize impactTelevision system detailsColor signal processing circuitsDigital videoComputer graphics (images)
A system for detecting picture freeze within a video signal wherein a video signal processing portion for connection to a video signal source comprising digital video luma samples and digital video clock and timing signals separately detects motion across the vertical axis of the video picture and across the horizontal axis of the video picture and wherein a frozen picture indicating portion connected to the output of the video signal processing portion determines the number of motionless video picture frames occurring since the last video picture frame in which motion was detected to indicate picture freeze when the number of motionless video picture frames reaches a predetermined value. A noise reduction threshold signal is applied to the video signal processing portion to distinguish motion from background noise in a video signal.
Owner:EVERTZ MICROSYSTEMS LTD
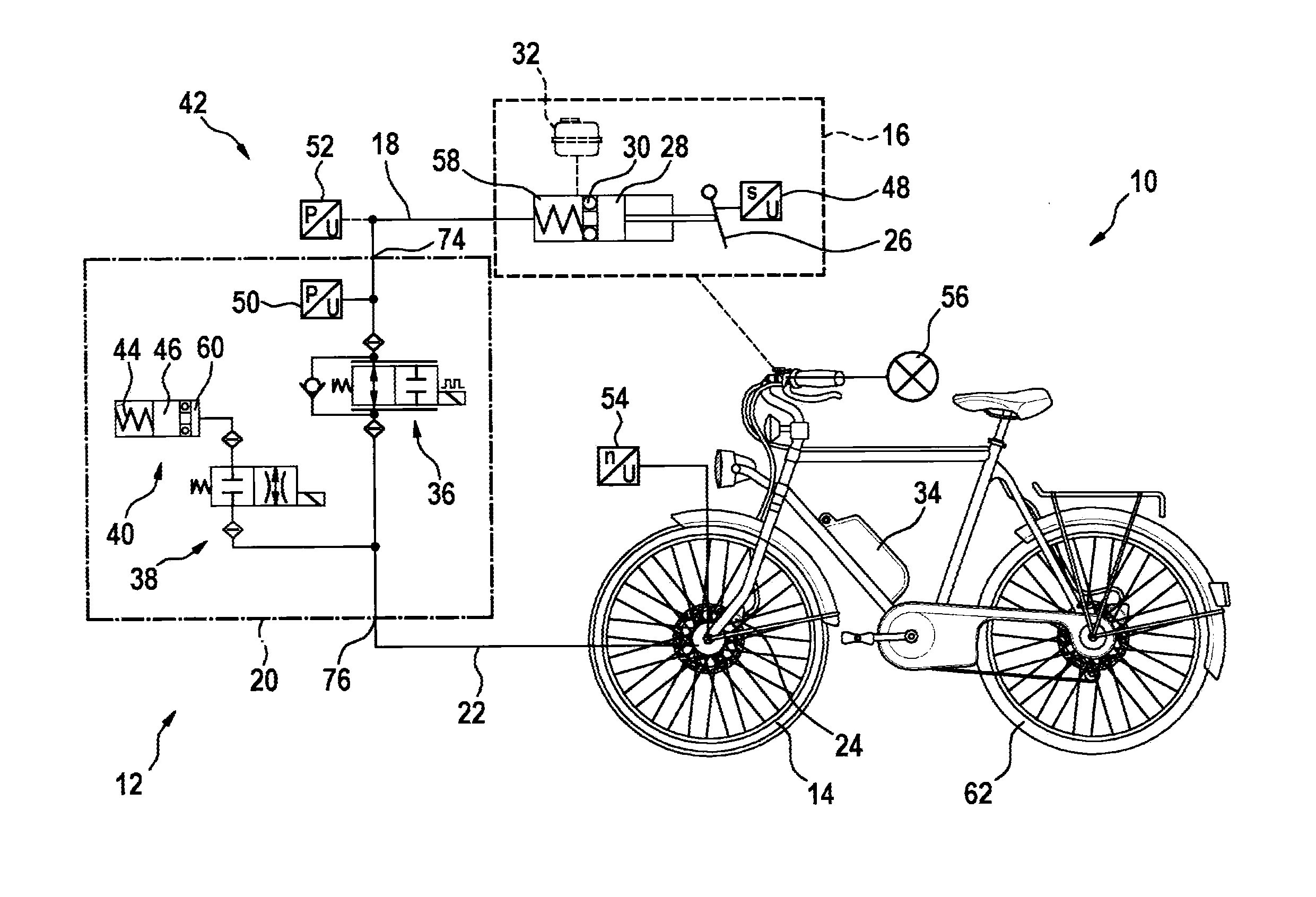
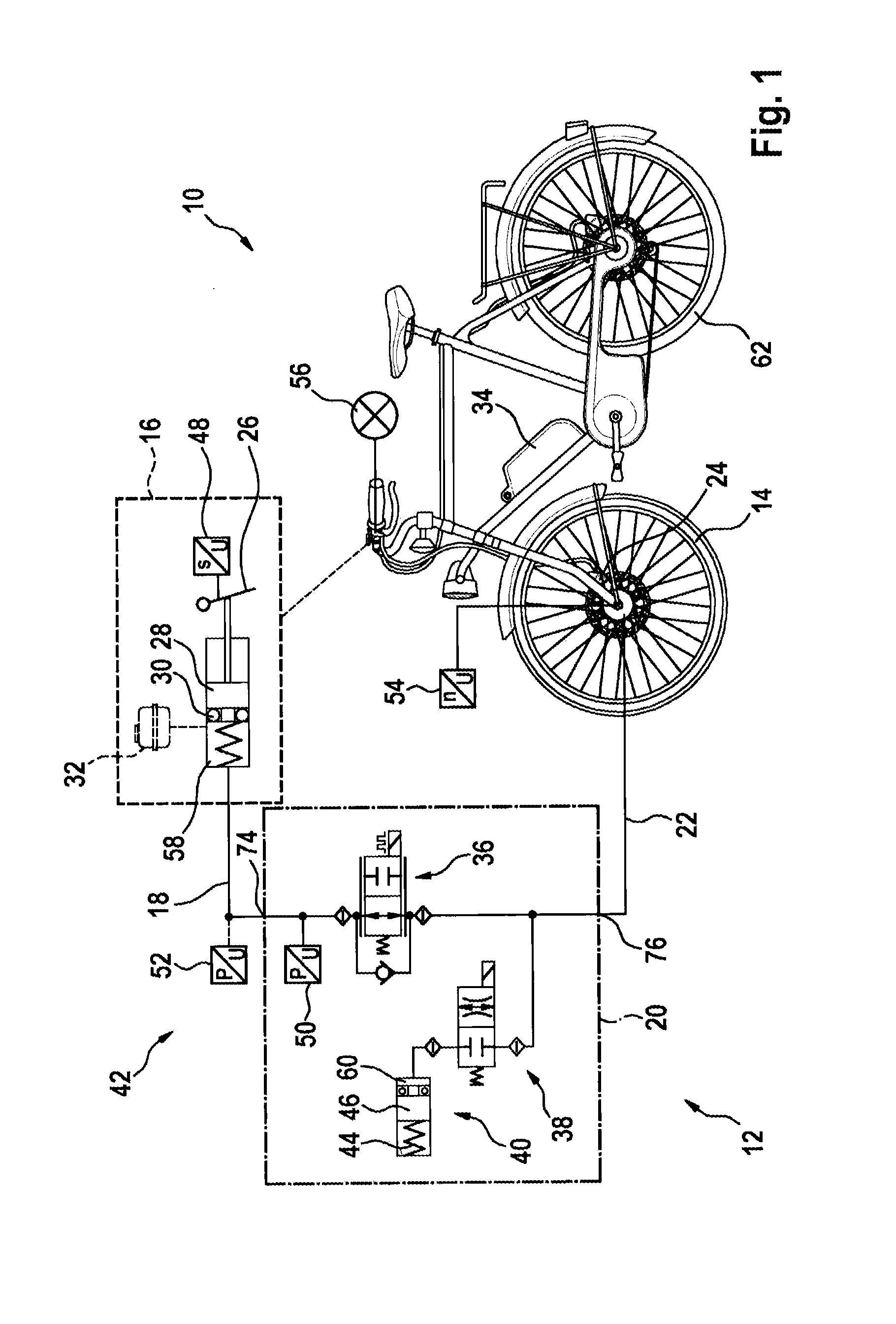
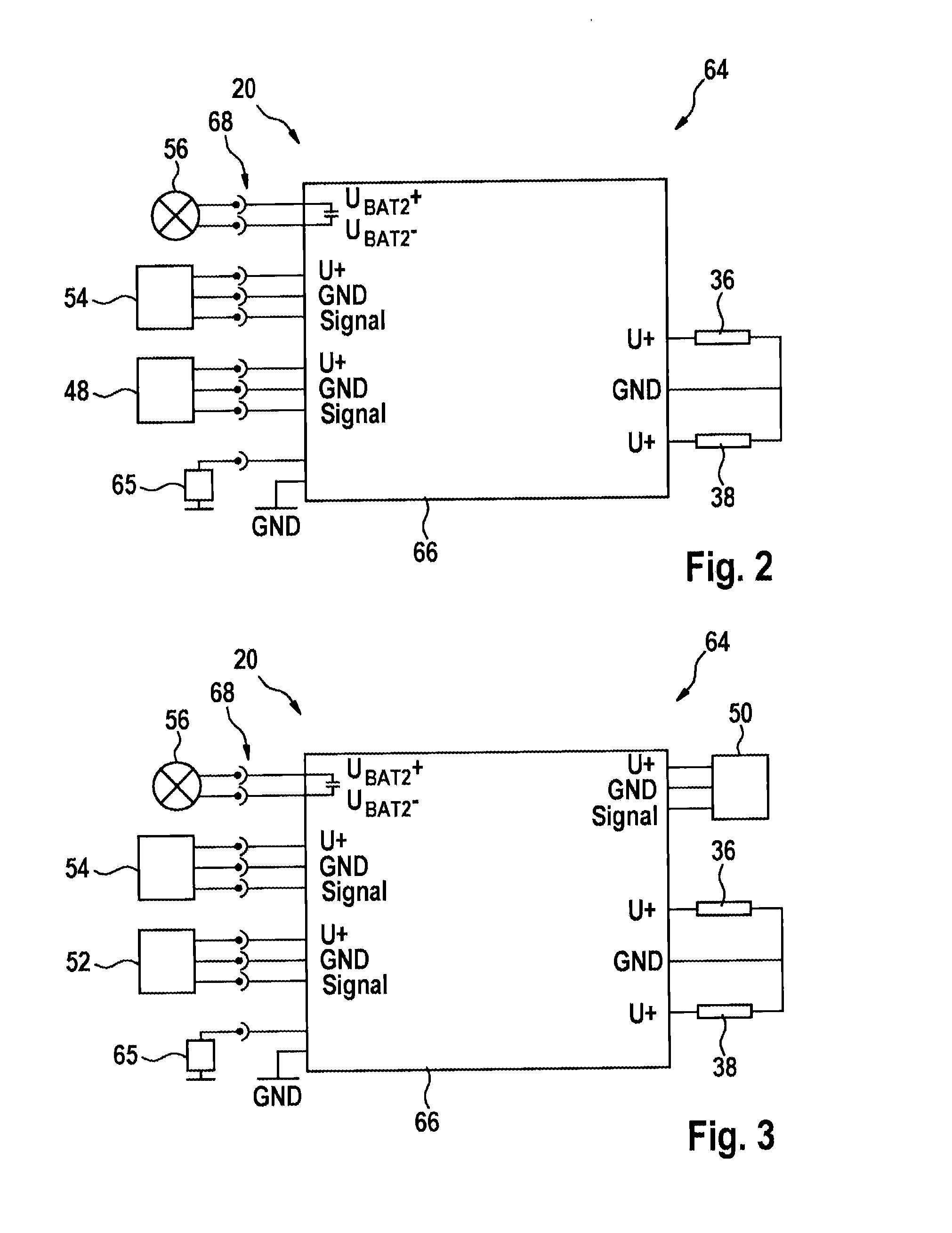
Hydraulic module for an antilock braking system for a two-wheeled vehicle
InactiveUS20150329094A1Inexpensive and easy to installReduce weightCycle brakesBraking systemsInlet valveEngineering
A hydraulic module for a hydraulic antilock braking system for a two-wheeled vehicle, the hydraulic module including a first hydraulic connection for connecting the hydraulic module by a hydraulic line to a brake-actuating device; a second hydraulic connection for connecting the hydraulic module by a hydraulic line to a wheel brake; an inlet valve for connecting and disconnecting the first hydraulic connection to / from the second hydraulic connection; an outlet valve for connecting and disconnecting a pressure accumulator to / from the second hydraulic connection; and a housing in which the inlet valve and the outlet valve are accommodated and which provides the first hydraulic connection and the second hydraulic connection.
Owner:ROBERT BOSCH GMBH
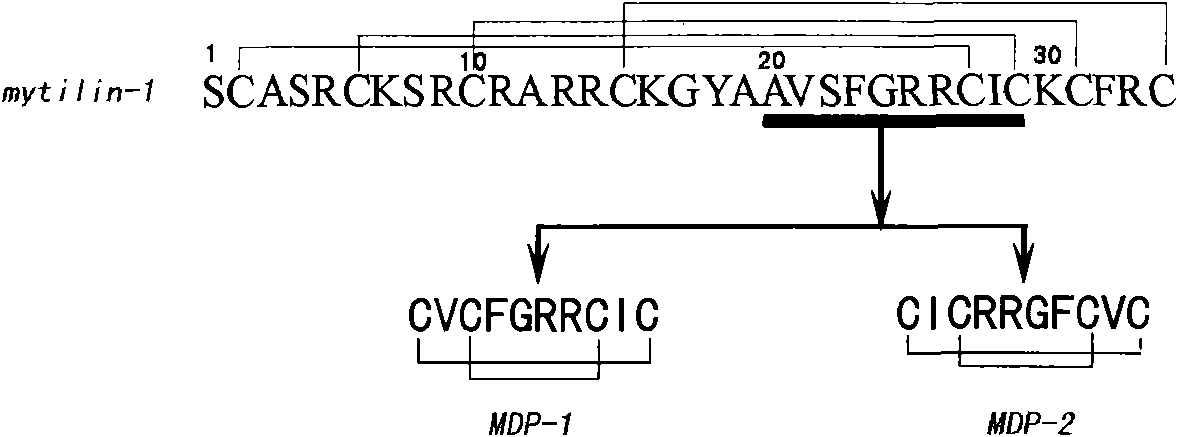
Artificially synthesized antimicrobial peptide, preparation method and application thereof
InactiveCN102079777ASmall molecular weightStable structureAntibacterial agentsAntimycoticsAntimicrobial peptidesGram-positive bacterium
The invention discloses an artificially synthesized antimicrobial peptide, which belongs to the field of biological pharmaceutical technology. The amino acid sequence of the artificially synthesized antimicrobial peptide is Cys-Val-Cys-Phe-Gly-Arg-ARG-Cys-Ile-Cys or Cys-Ile-Cys-Arg-Arg-Gly-Phe-Cys-Val-Cys. The invention also discloses a method for artificially synthesizing the antimicrobial peptide and application of the artificially synthesized antimicrobial peptide in development of preparing medicaments for treating Gram-positive bacteria and Gram-negative bacteria or feed additives. Compared with the prior art, the artificially synthesized antimicrobial peptide has the following advantages and effects, such as small molecular weight as compared with the natural ones, stable structure, strong antimicrobial activity, short sequence, less disulfide bond number, low cost and the like.
Owner:ZHEJIANG OCEAN UNIV
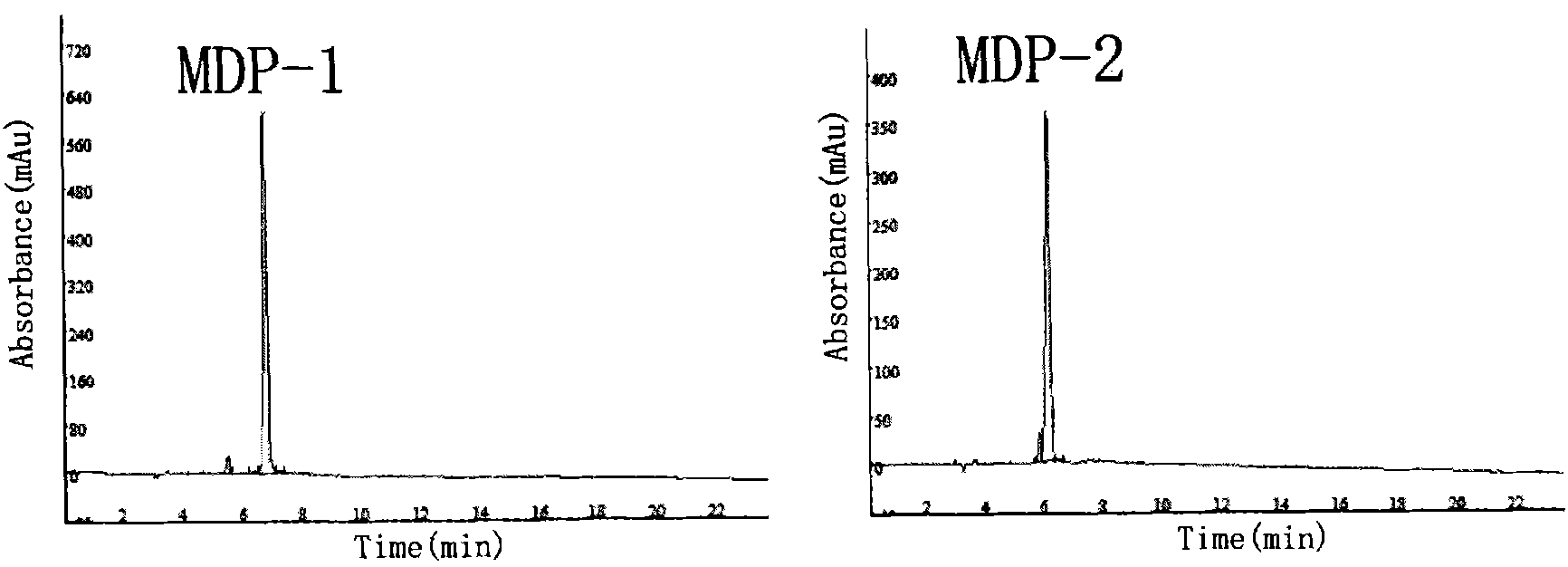
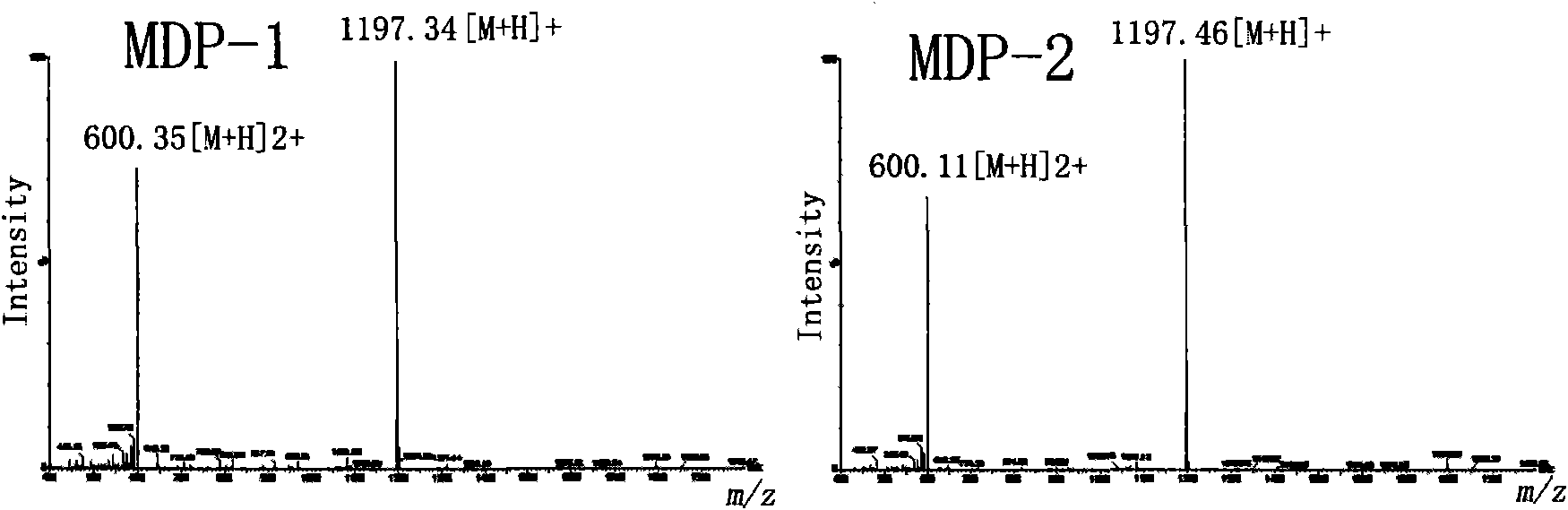
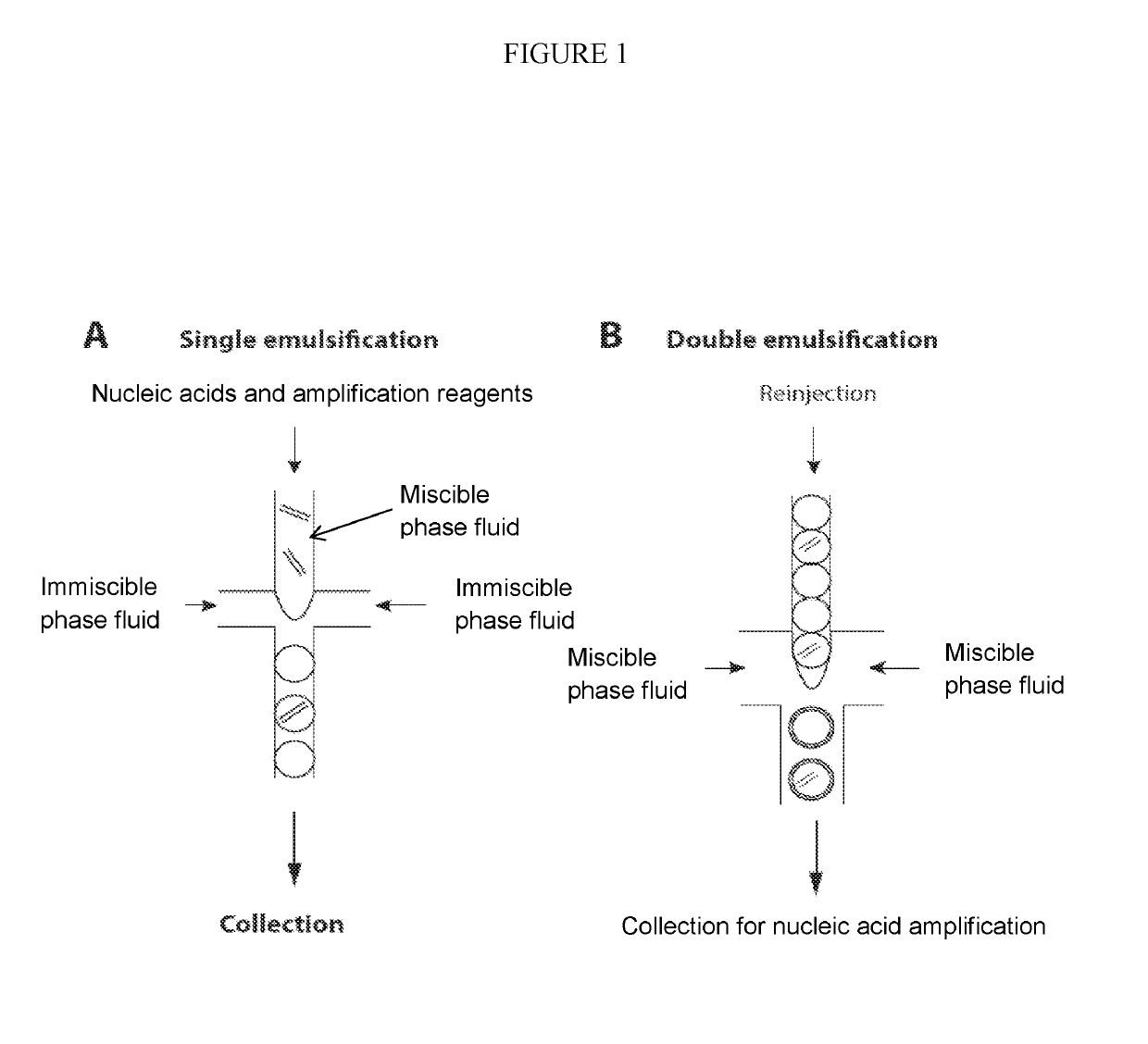
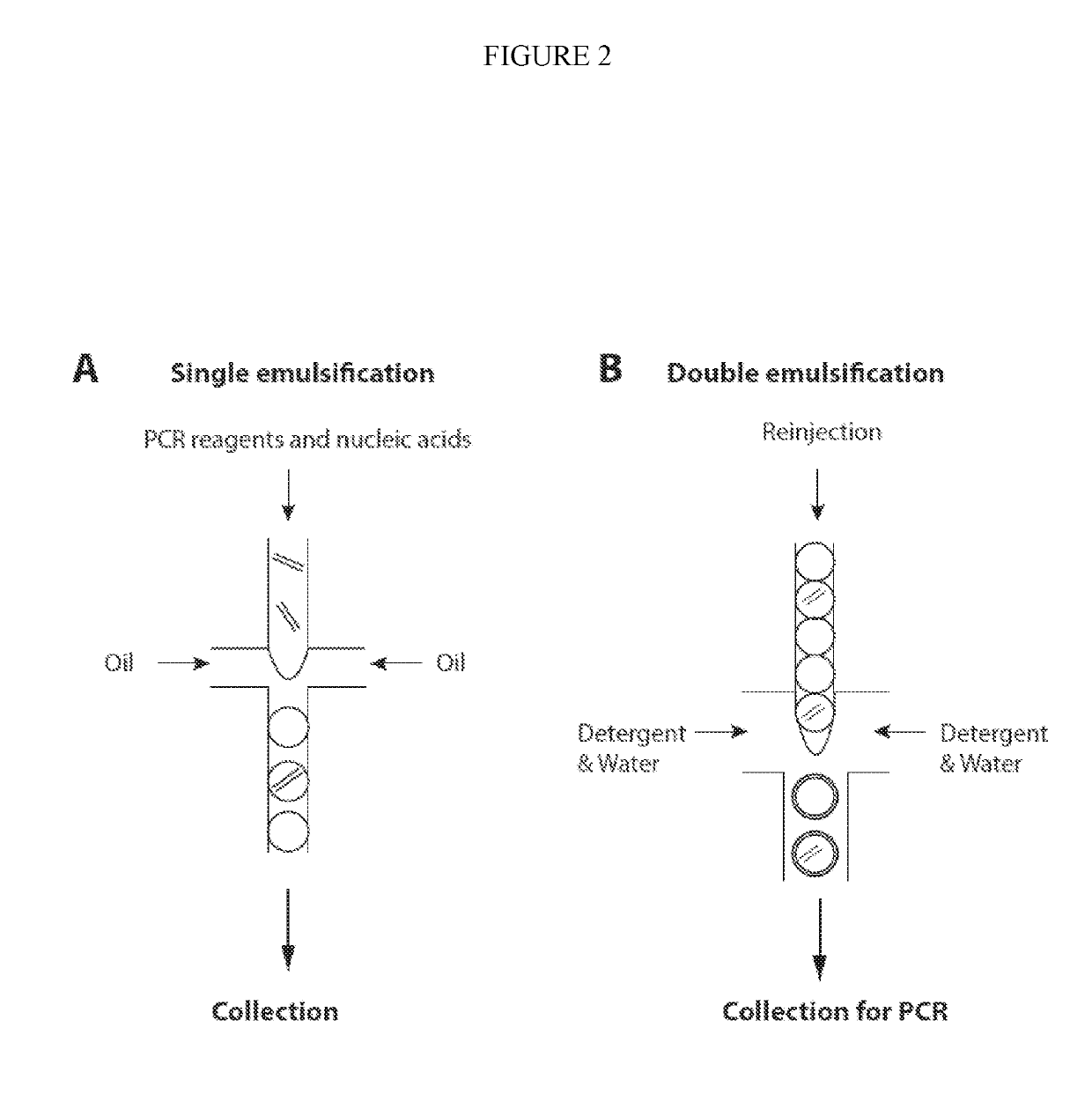
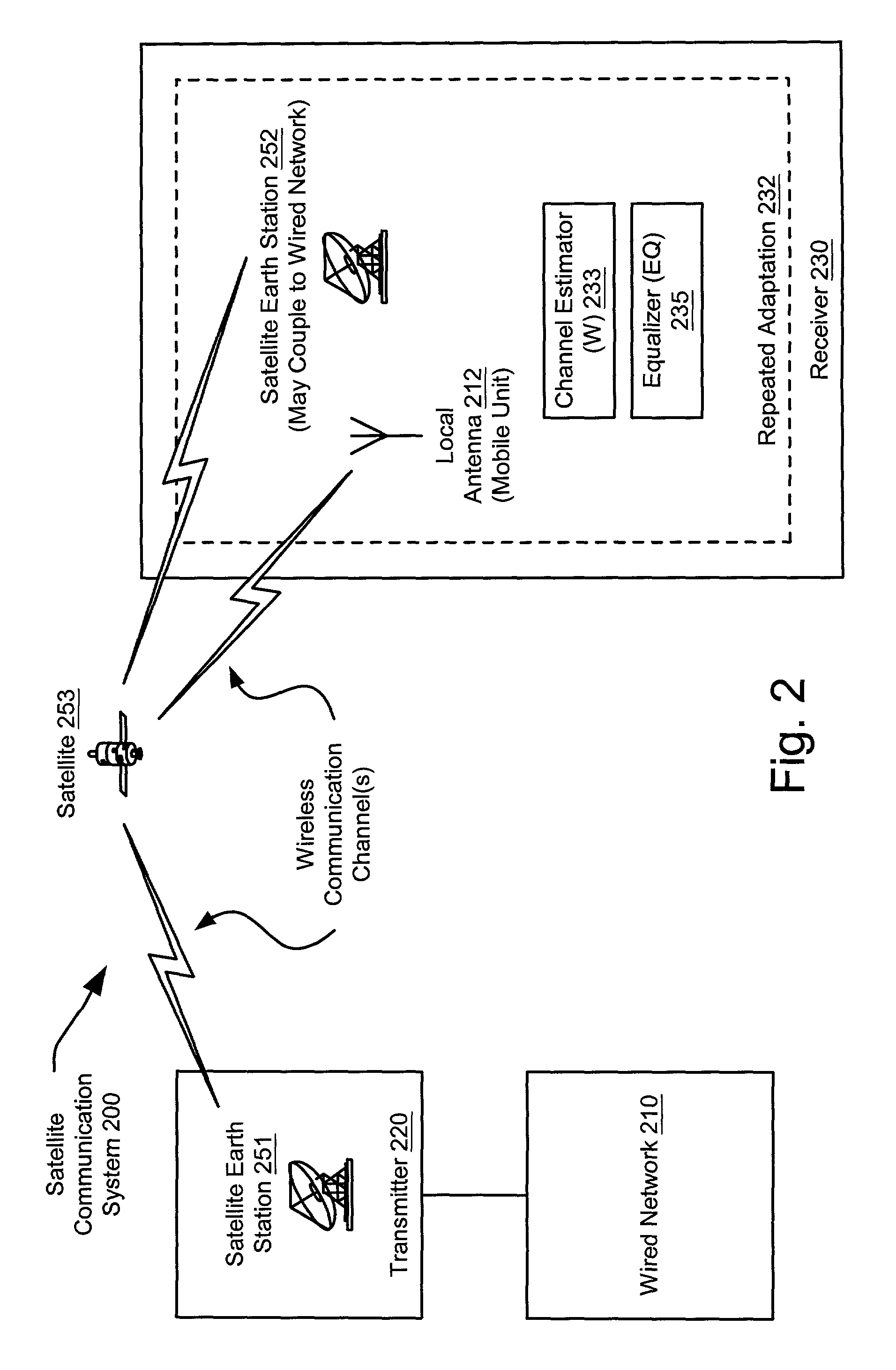
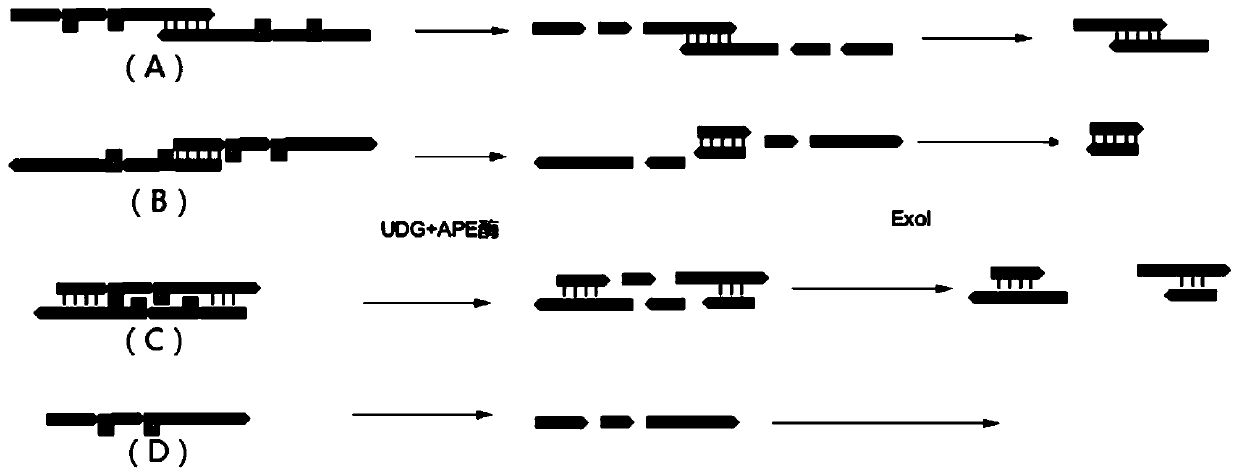
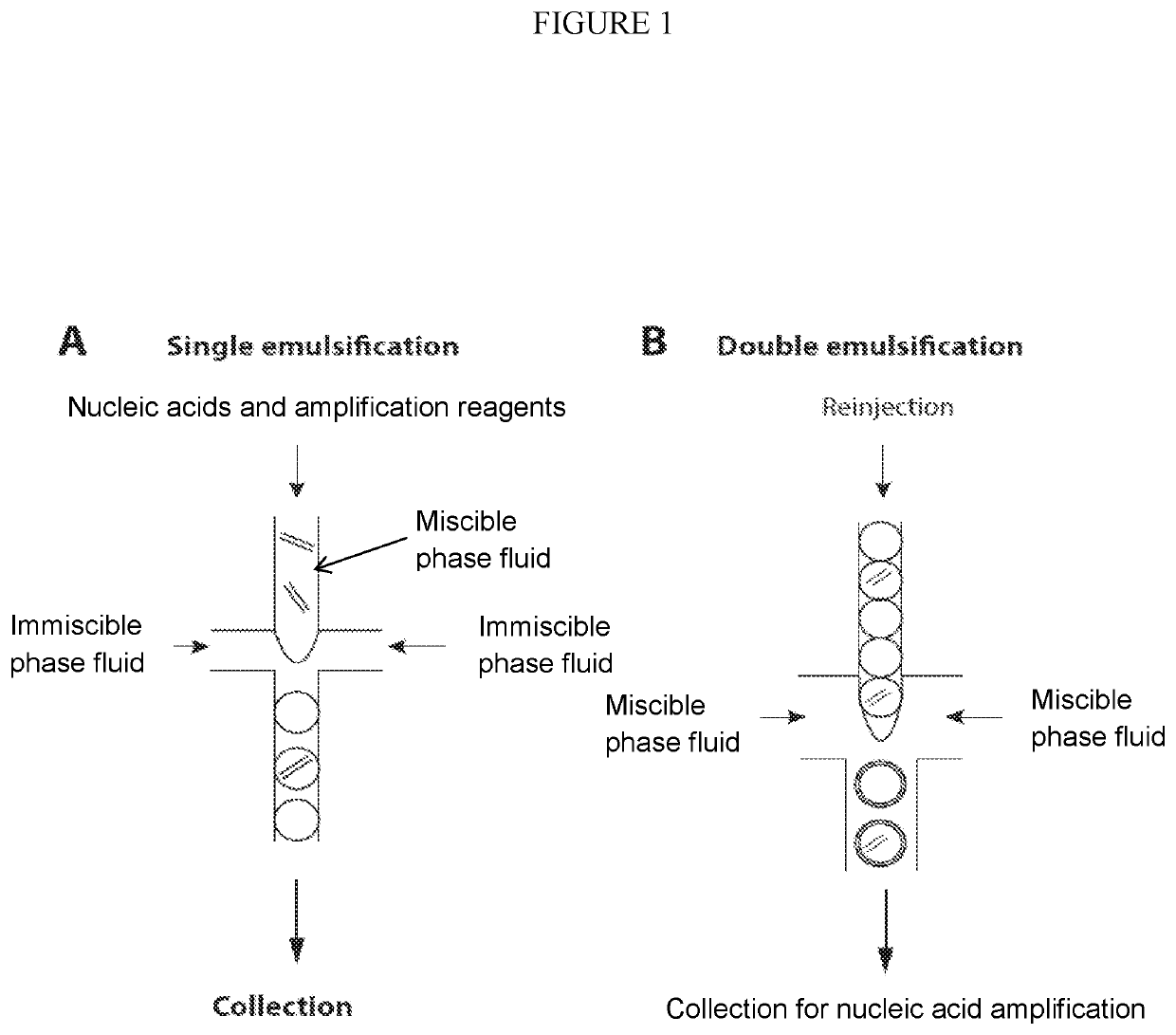
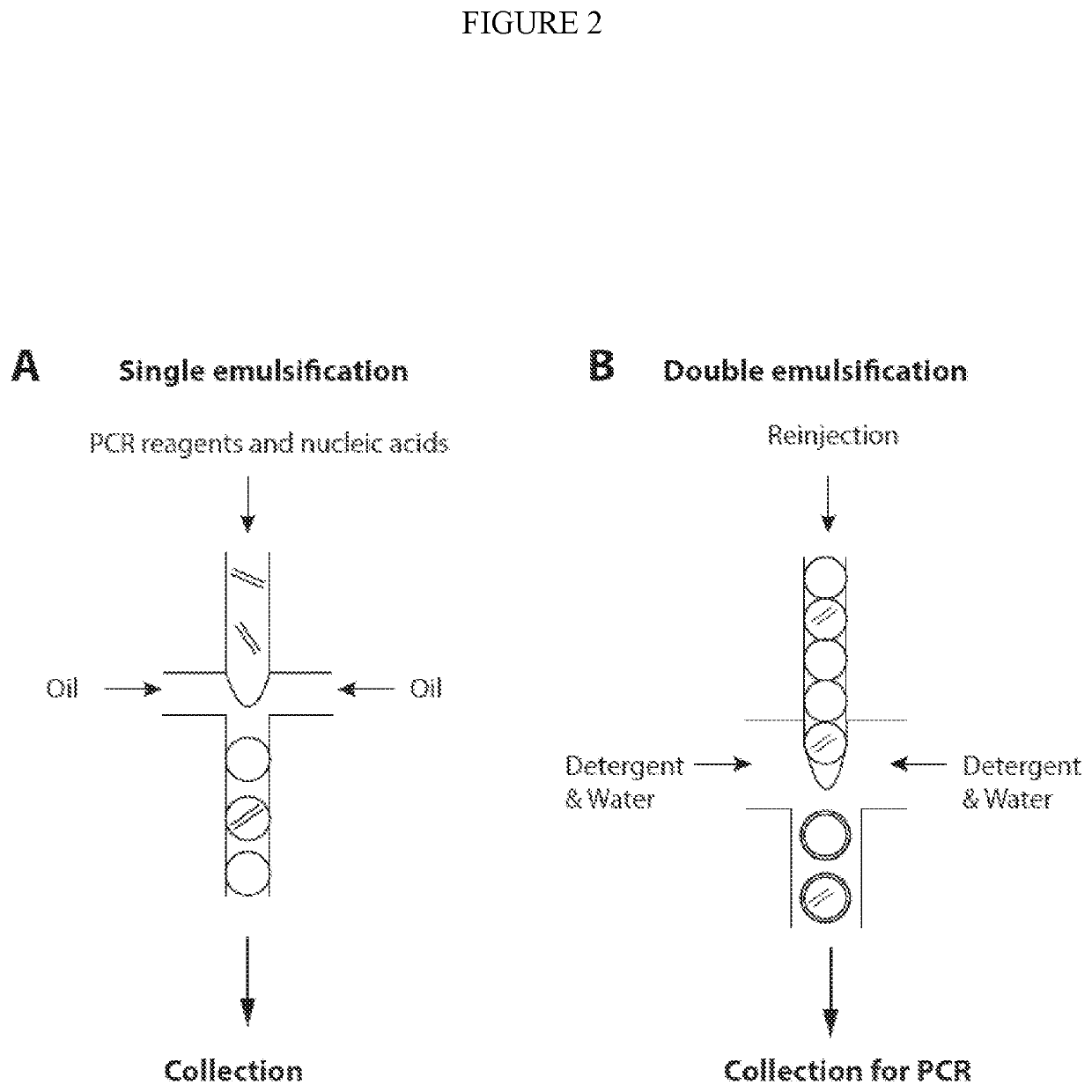
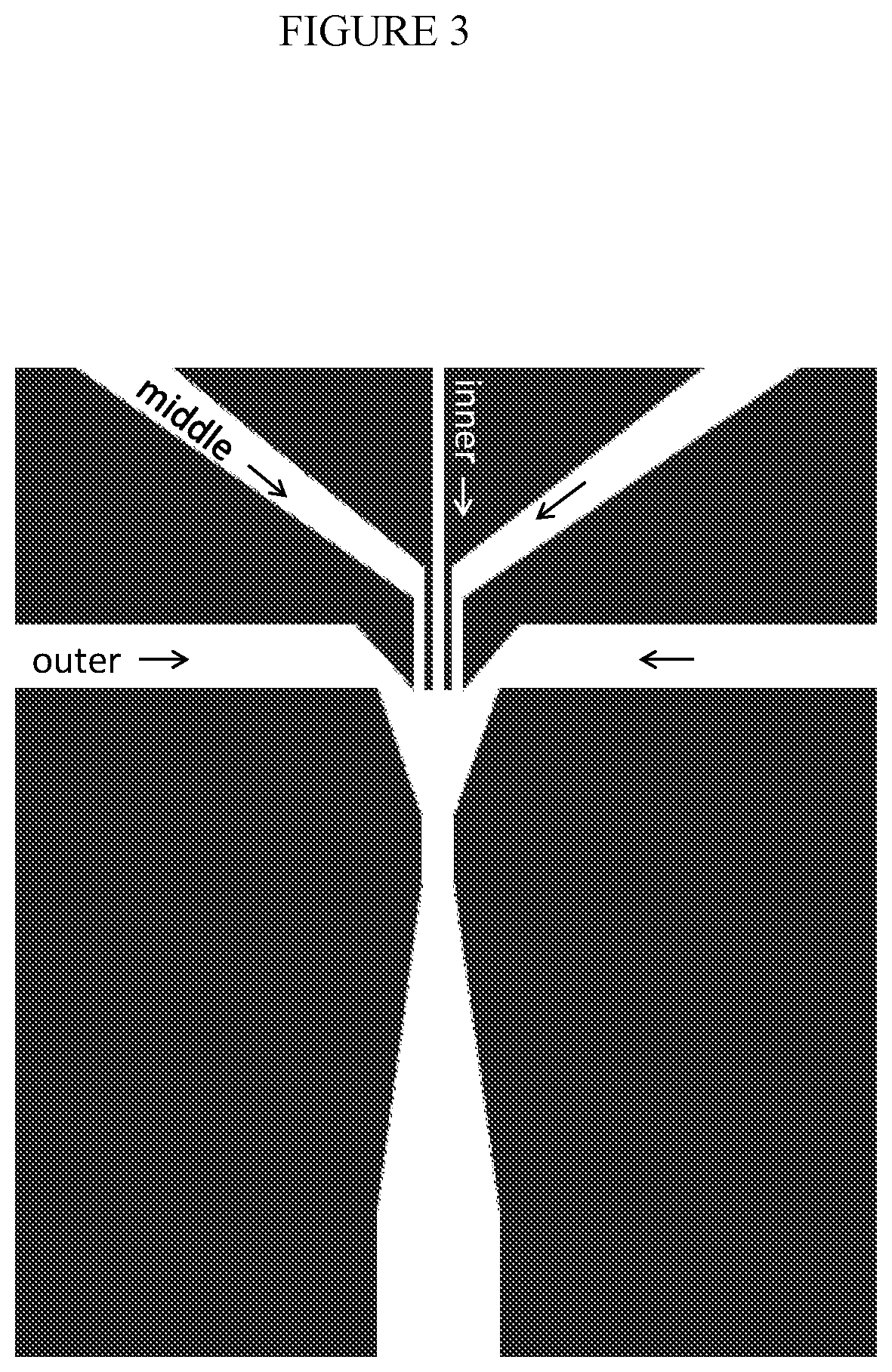
Combined multiple-displacement amplification and PCR in an emulsion microdroplet
ActiveUS20190218594A1Easy to identifySequencing is facilitatedHeating or cooling apparatusMicrobiological testing/measurementNucleic acid amplification techniqueVirus
The methods and systems described herein provide an improved emulsion droplet based nucleic acid amplification method, which allows nucleic acids contained in biological systems to be detected, quantitated and / or sorted based on their sequence as detected with nucleic acid amplification techniques, e.g., polymerase chain reaction (PCR). The nucleic acids can be free floating or contained within living or nonliving structures, including particles, viruses, and cells. The nucleic acids can include, e.g., DNA or RNA.
Owner:RGT UNIV OF CALIFORNIA
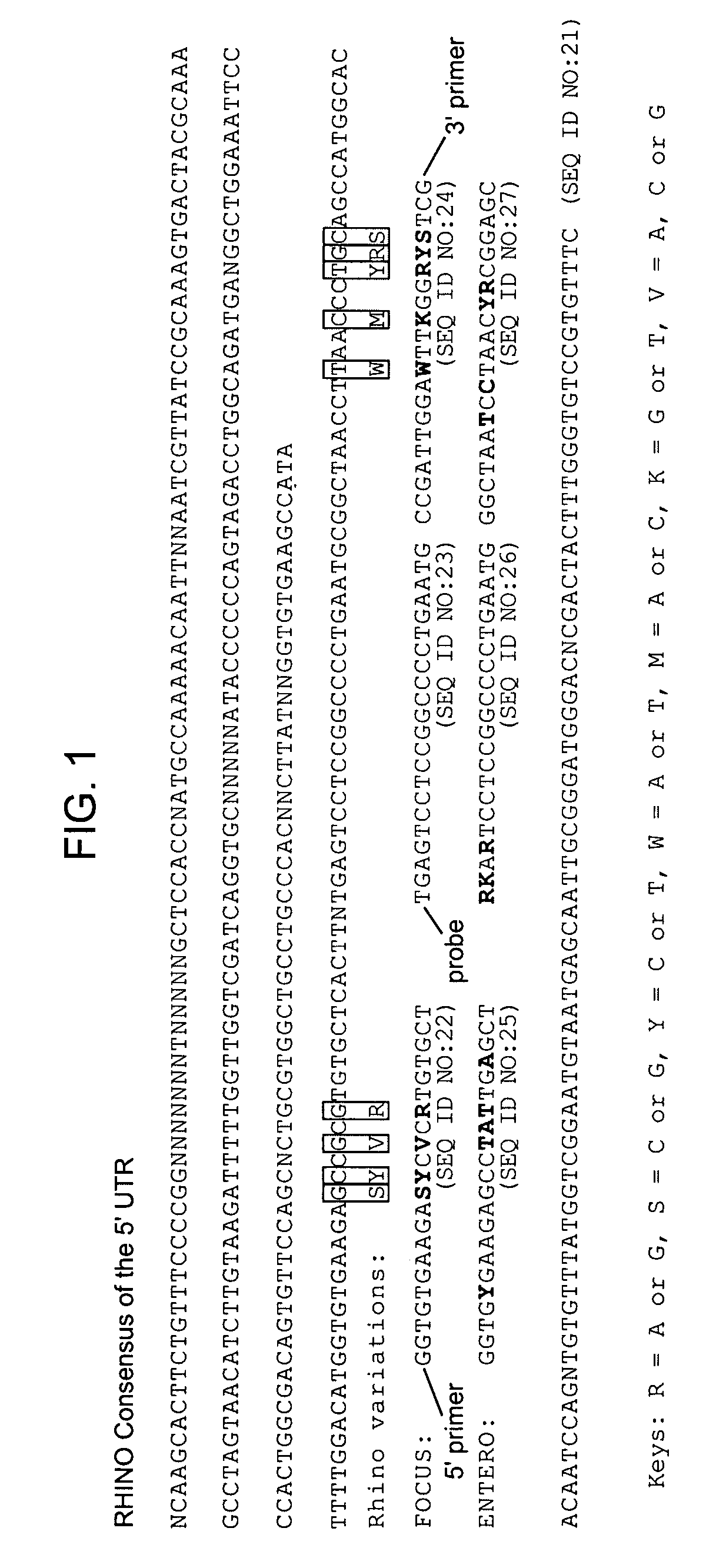
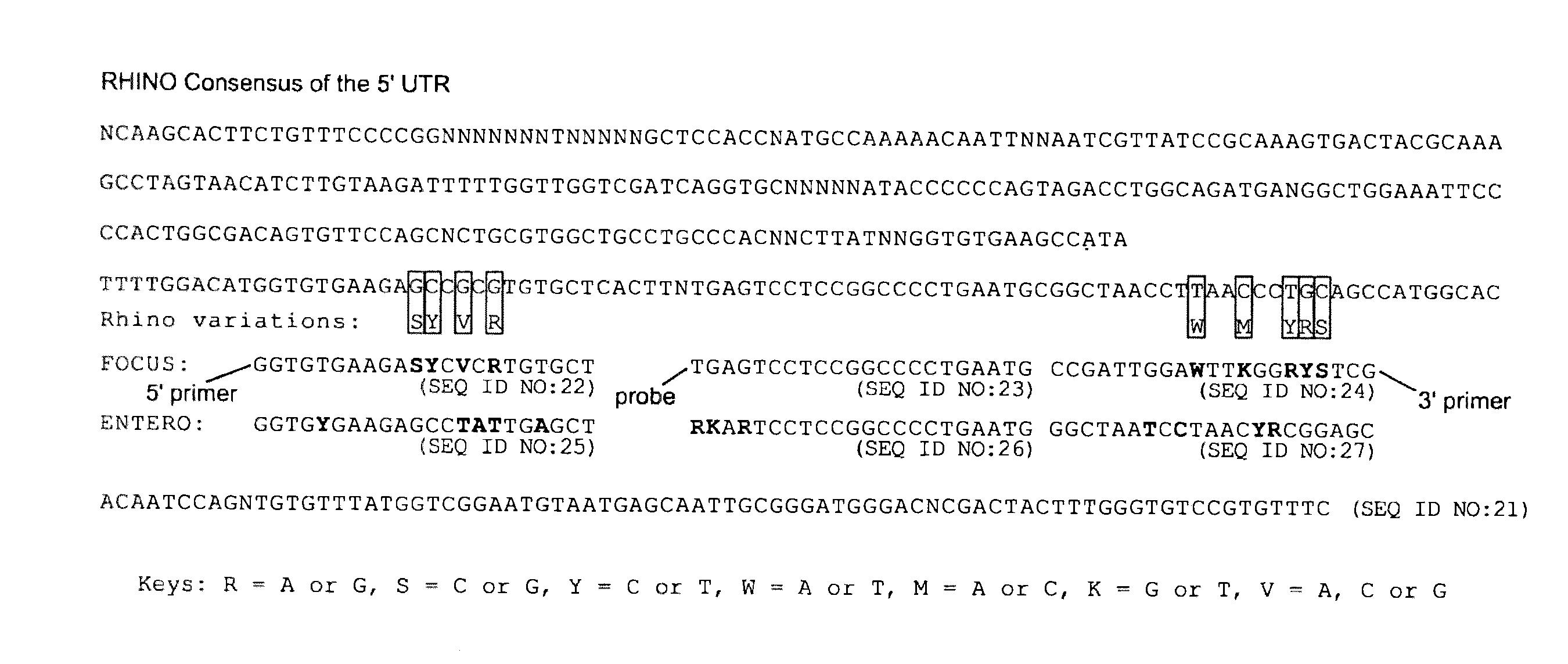
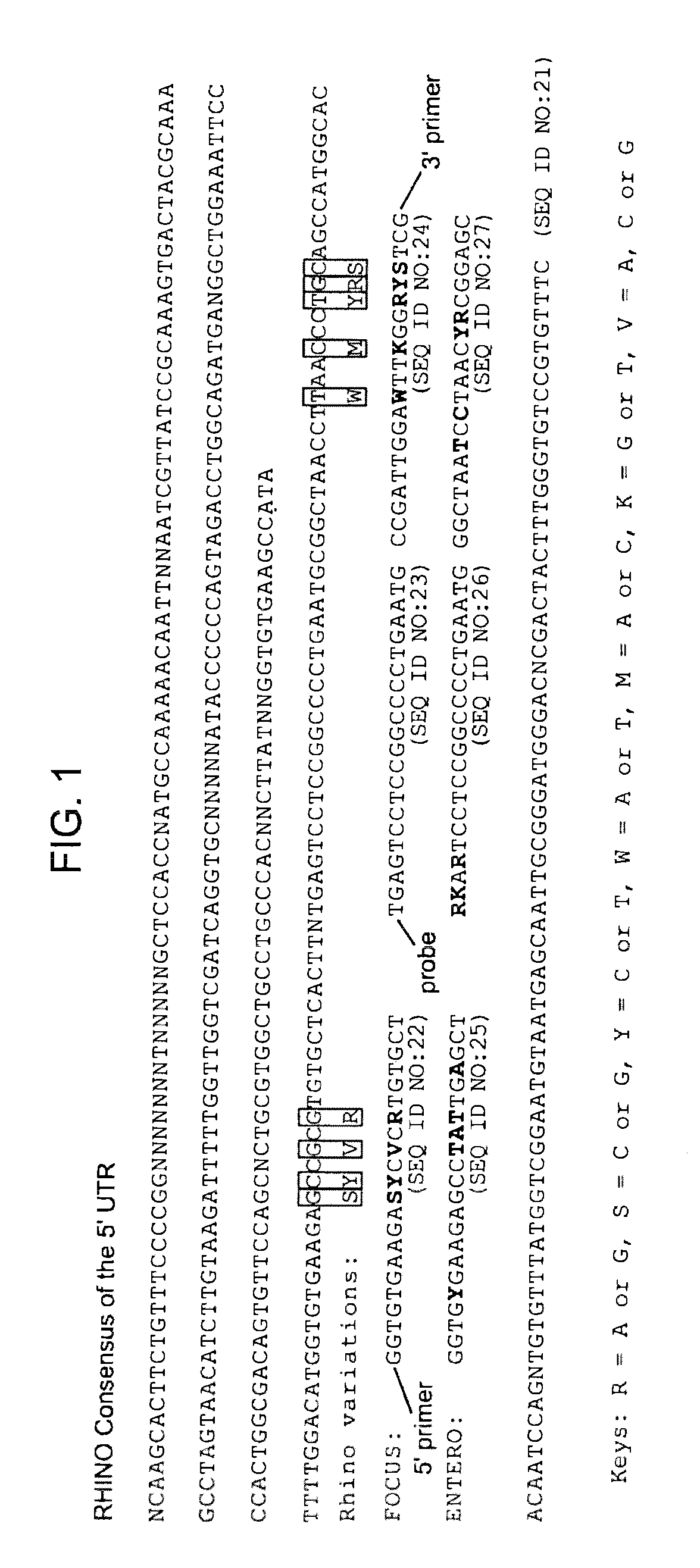
Methods and compositions for detecting rhinoviruses
The invention provides methods and compositions for rapid, sensitive, and highly specific nucleic acid-based (e.g., DNA based) detection of human rhinovirus (HRV) in a sample. In general, the methods involve detecting a target nucleic acid having a target sequence of a conserved 5′ untranslated region of the HRV genome. The invention also features compositions, including primers, probes, and kits, for use in the methods of the invention.
Owner:FOCUS DIAGNOSTICS
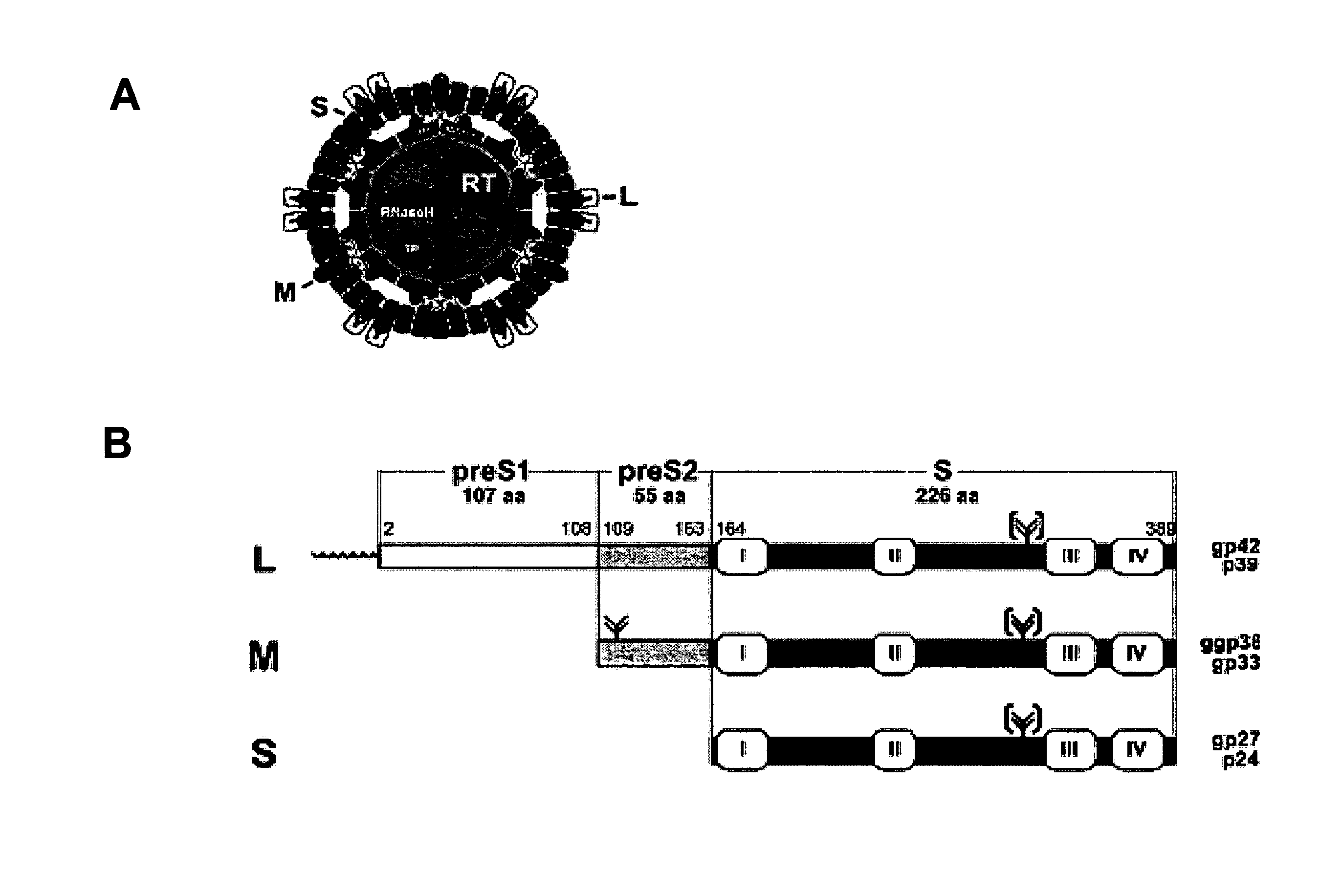
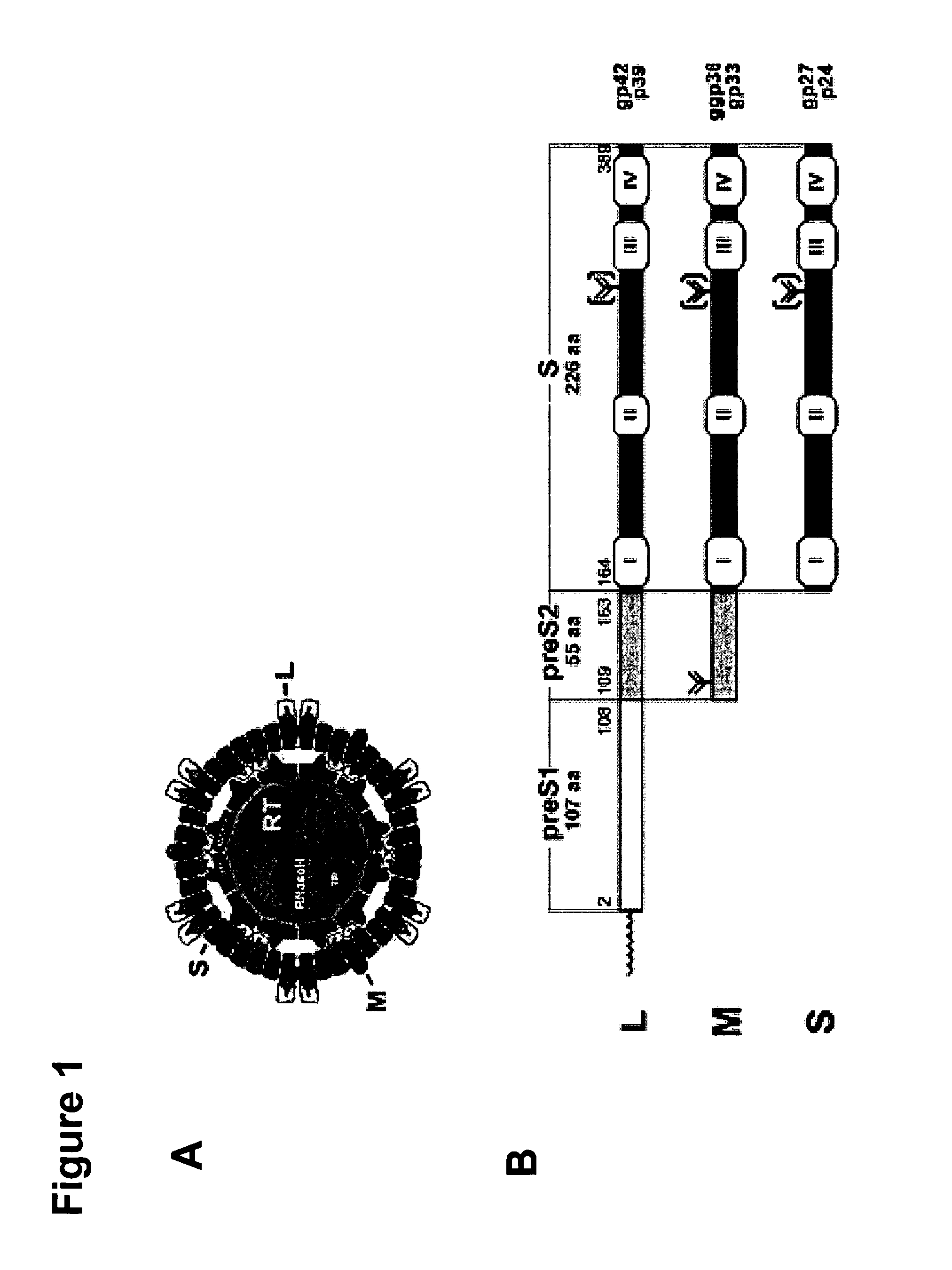
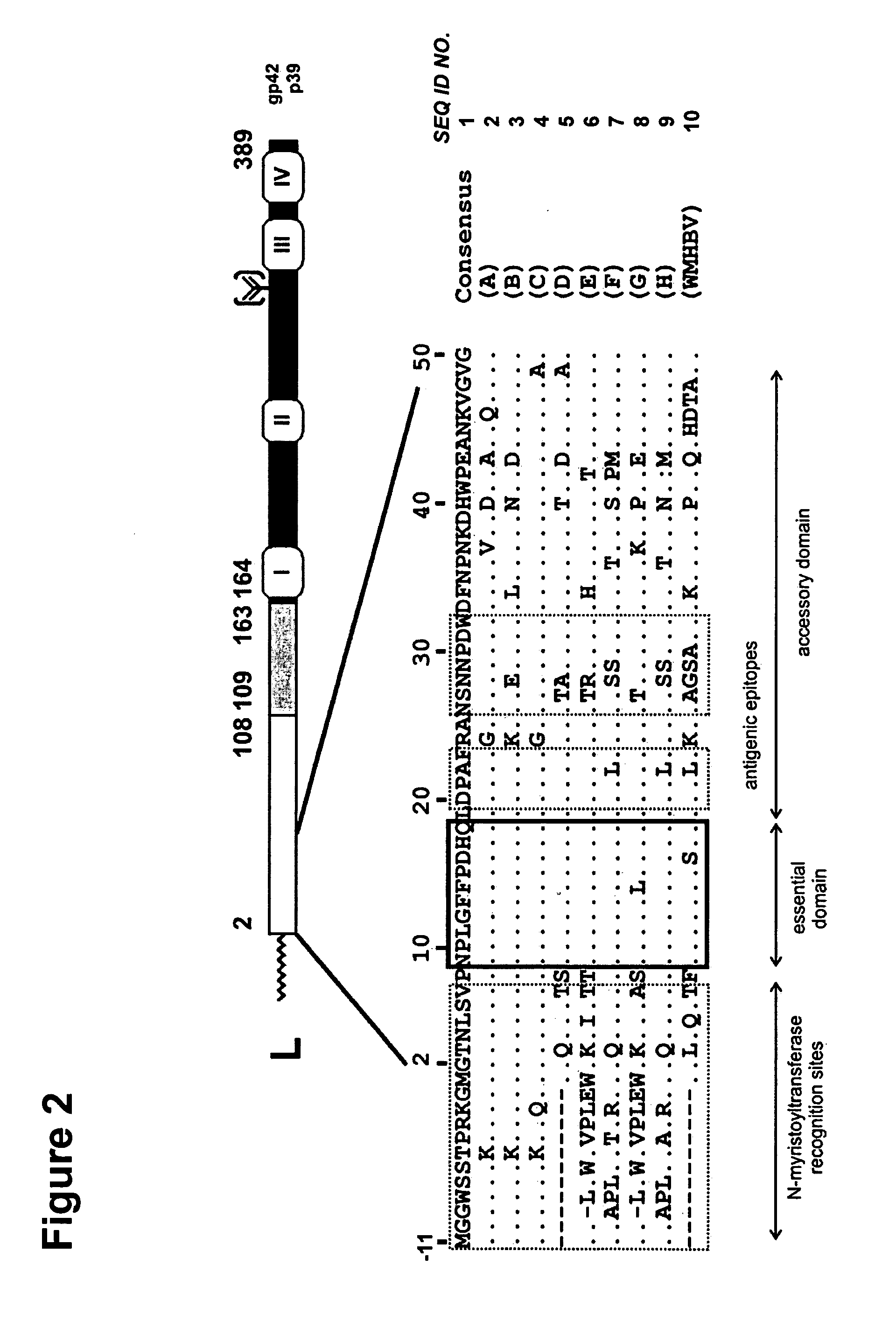
Hydrophobic Modified Pres-Derived Peptides of Hepatitis B Virus (HBV) and Their Use as Vehicles for the Specific Delivery of Compounds to the Liver
ActiveUS20110027183A1Low immunogenicityShort sequenceOrganic active ingredientsVirusesDiseaseCompound specific
The present invention relates to hydrophobic modified preS-derived peptides of hepatitis B virus (HBV) which are versatile vehicles for the specific delivery of compounds to the liver, preferably to hepatocytes, in vitro as well as in vivo. Any kind of compound, but in particular drugs, such as interferons, viral reverse transcriptase inhibitors or core assembly inhibitors, and / or labels can be specifically targeted to the liver and so be enriched in the liver. This liver targeting can further be used for the targeted diagnosis, prevention and / or treatment of liver diseases or disorders, such as hepatitis, malaria, hepatocellular carcinoma (HCC), as well as for the prevention of HAV, HBV, HCV and / or HDV infection. The present invention relates to pharmaceutical compositions comprising said hydrophobic modified preS-derived peptide(s) and the compound(s) to be specifically delivered to the liver. The present invention furthermore relates to a method for the combined treatment of a liver disease and the prevention of HAV, HBV, HCV and / or HDV infection. The present invention relates also to the use of the preS-derived peptides in gene therapy and the delivery of immunogenic epitopes for hepatocyte-mediated antigen presentation to activate liver-directed immunological responses.
Owner:UNIVERSITY OF HEIDELBERG
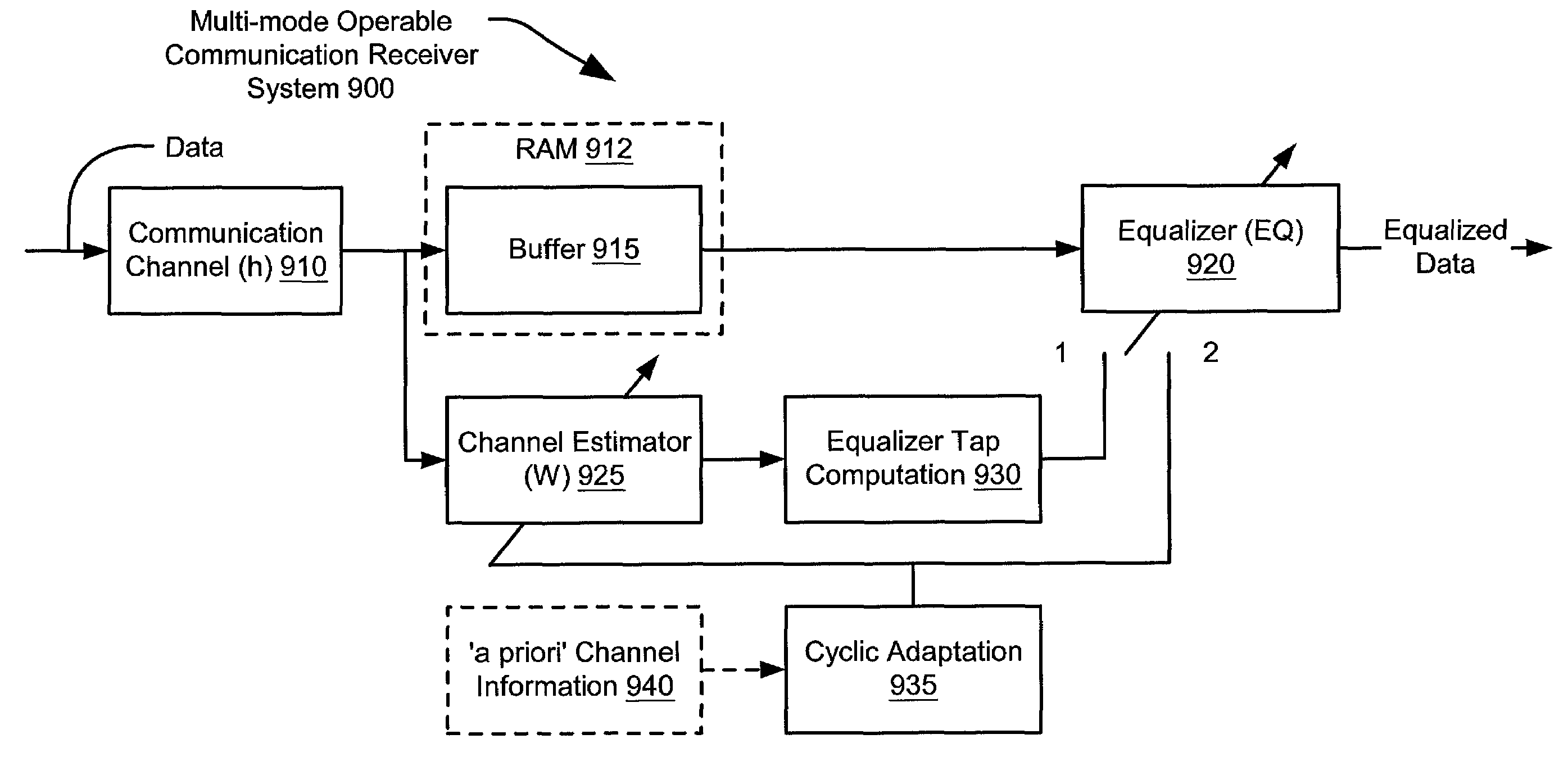
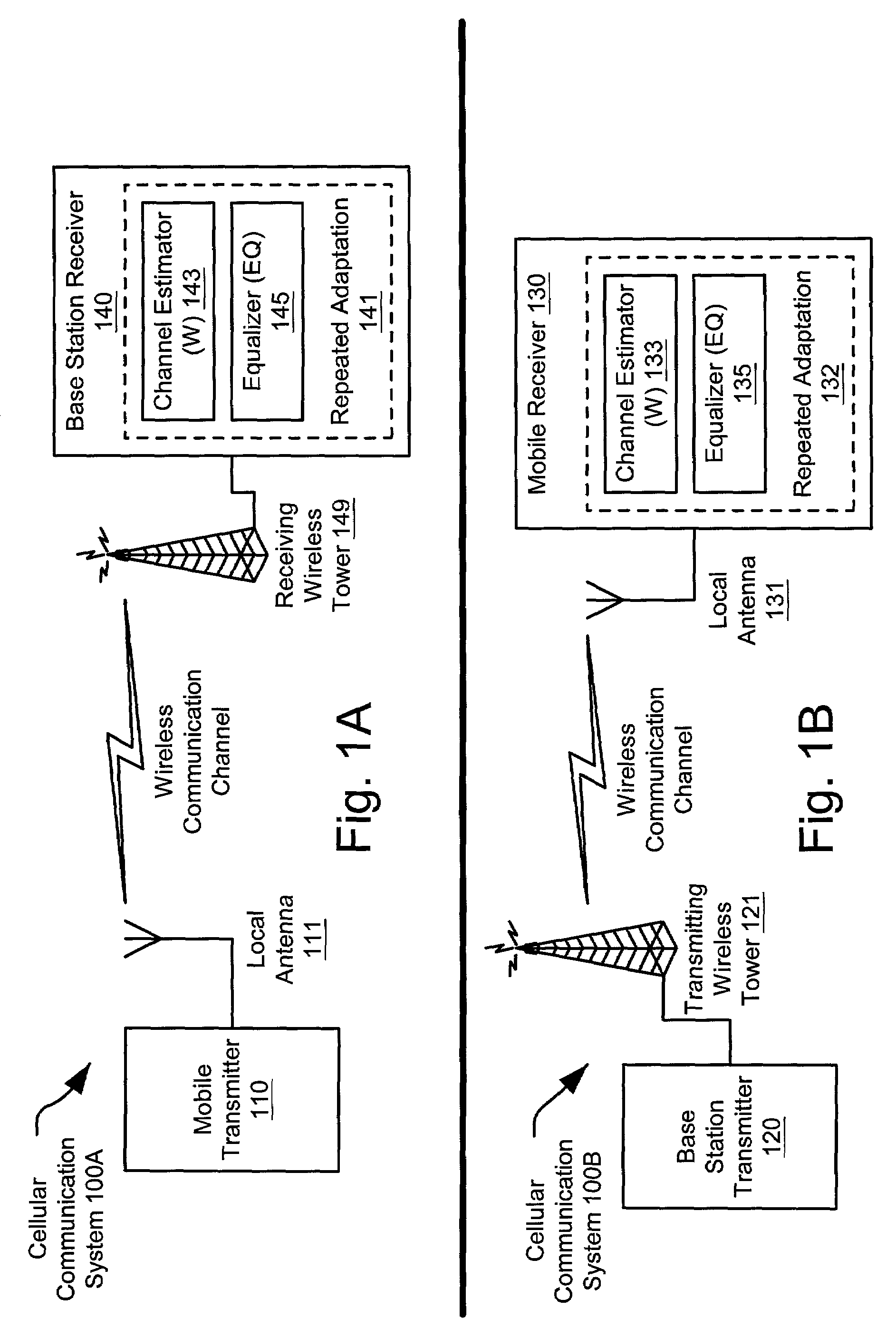
Channel estimation and/or equalization using repeated adaptation
InactiveUS7099409B2Convergence can be speededReduce in quantityAmplitude-modulated carrier systemsAmplitude demodulationAlgorithmEqualization
A novel approach of repeated adaptation is provided that can be applied to either one or both of channel estimation and / or equalization. From an incoming data packet that includes data and a training sequence, a modified data packet is generated that includes the data, the training sequence, and at least one additional copy of the training sequence. From the format of this modified data packet, the same training sequence can be used over and over again a desired number of times to perform channel estimation and subsequent calculation of equalizer tap coefficients. Alternatively, the same training sequence can be used over and over again a desired number of times to converge the equalizer coefficient taps directly without doing any preliminary channel estimation. Generally, either of these approaches can be characterized as a cyclic adaptation operation that provides improved performance without incurring any reduction in throughput of the communication channel.
Owner:AVAGO TECH WIRELESS IP SINGAPORE PTE
Identification method of rice bacterial leaf blight resistance and application of miRNA397a genetic locus
ActiveCN103184286AShort sequenceNot easy to degradeMicrobiological testing/measurementMiRNA GeneResistant genes
The invention discloses an identification method of rice bacterial leaf blight resistance and an application of miRNA397a genetic locus, and belongs to the field of biotechnology. The identification method comprises the following steps: inoculating paddy rice to be detected and infected paddy rice; separating miRNA genes of the paddy rice to be detected and the infected paddy rice; detecting the expressions of the miRNA genes; and determining whether the paddy rice to be detected is resistant to bacterial leaf blight. The invention further discloses the application of the miRNA397a genetic locus in identification of rice bacterial leaf bright resistance. The invention uses the transcription of the miRNA397a gene locus to identify whether paddy rice is resistant to bacterial leaf blight, and achieves success. The transcription of the miRNA397a gene locus provided by the invention is detection based on expression level, so as to avoid misjudgment caused by bacterial leaf blight resistance gene silencing; and the identification method provided by the invention does not use the conventional DNA linkage tracer method, so as to avoid misjudgment caused by untightness of DNA linkage, improve the detection accuracy and avoid unnecessary production loss.
Owner:JIANGHAN UNIVERSITY
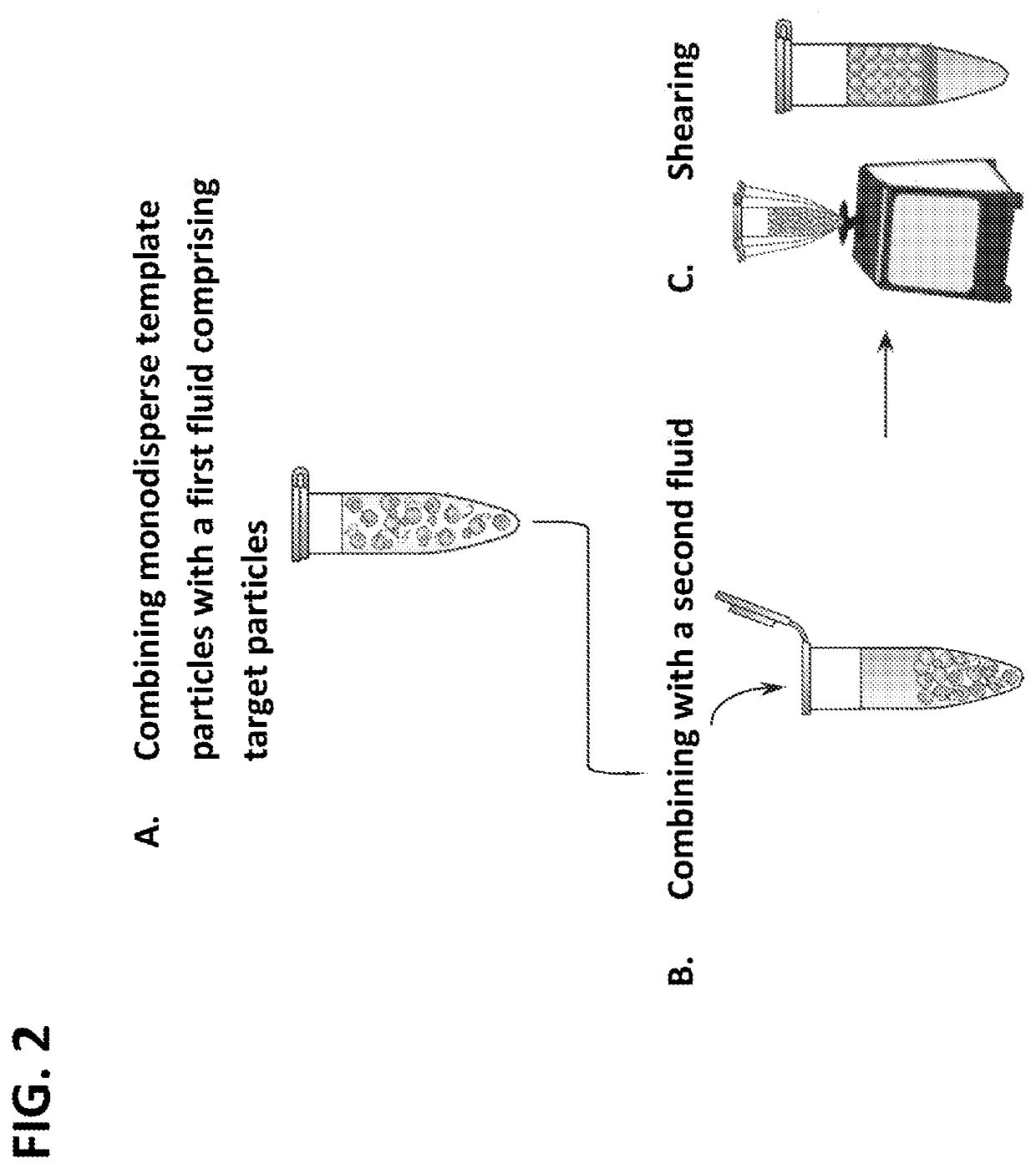
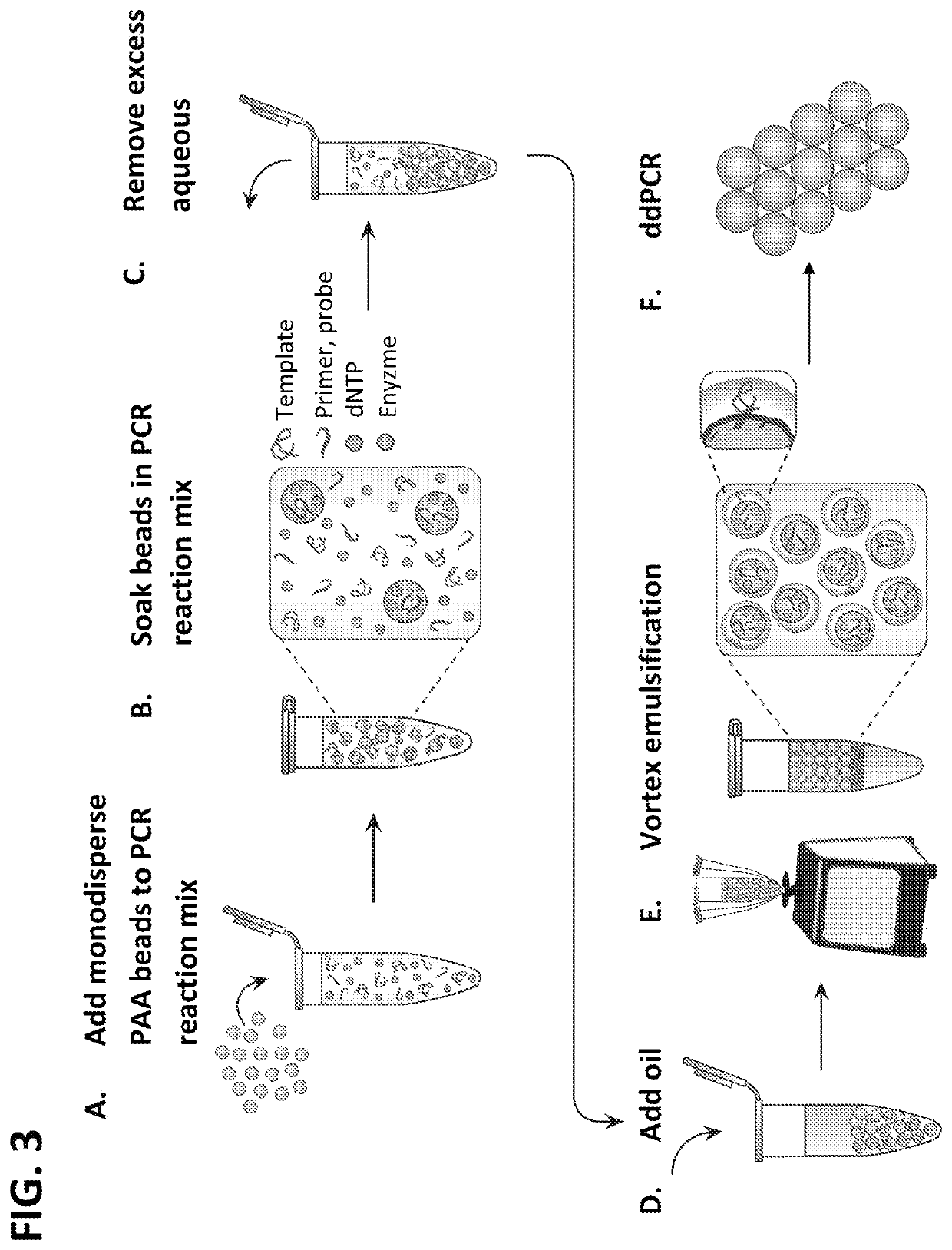
Method of generating monodisperse emulsions
PendingUS20200261879A1Efficiently obtainedEasy to detectTransportation and packagingMicrobiological testing/measurementEmulsion dropletVesicle
The methods described herein, referred to as particle-templated emulsification (PTE), provide an improved approach for generating a monodisperse emulsion that encapsulates target particles of interest without requiring the use of a microfluidic device. Monodisperse droplets may be effectively obtained by using monodisperse particles to template the formation of droplets, which can include, e.g., monodisperse single-emulsion droplets, multiple-emulsion droplets, or Giant Unilamellar Vesicles (GUV), without destroying the integrity of the droplets.
Owner:RGT UNIV OF CALIFORNIA
Methods and compositions for detecting rhinoviruses
The invention provides methods and compositions for rapid, sensitive, and highly specific nucleic acid-based (e.g., DNA based) detection of human rhinovirus (HRV) in a sample. In general, the methods involve detecting a target nucleic acid having a target sequence of a conserved 5′ untranslated region of the HRV genome. The invention also features compositions, including primers, probes, and kits, for use in the methods of the invention.
Owner:FOCUS DIAGNOSTICS
An intelligent community safety monitoring system and a safety monitoring method
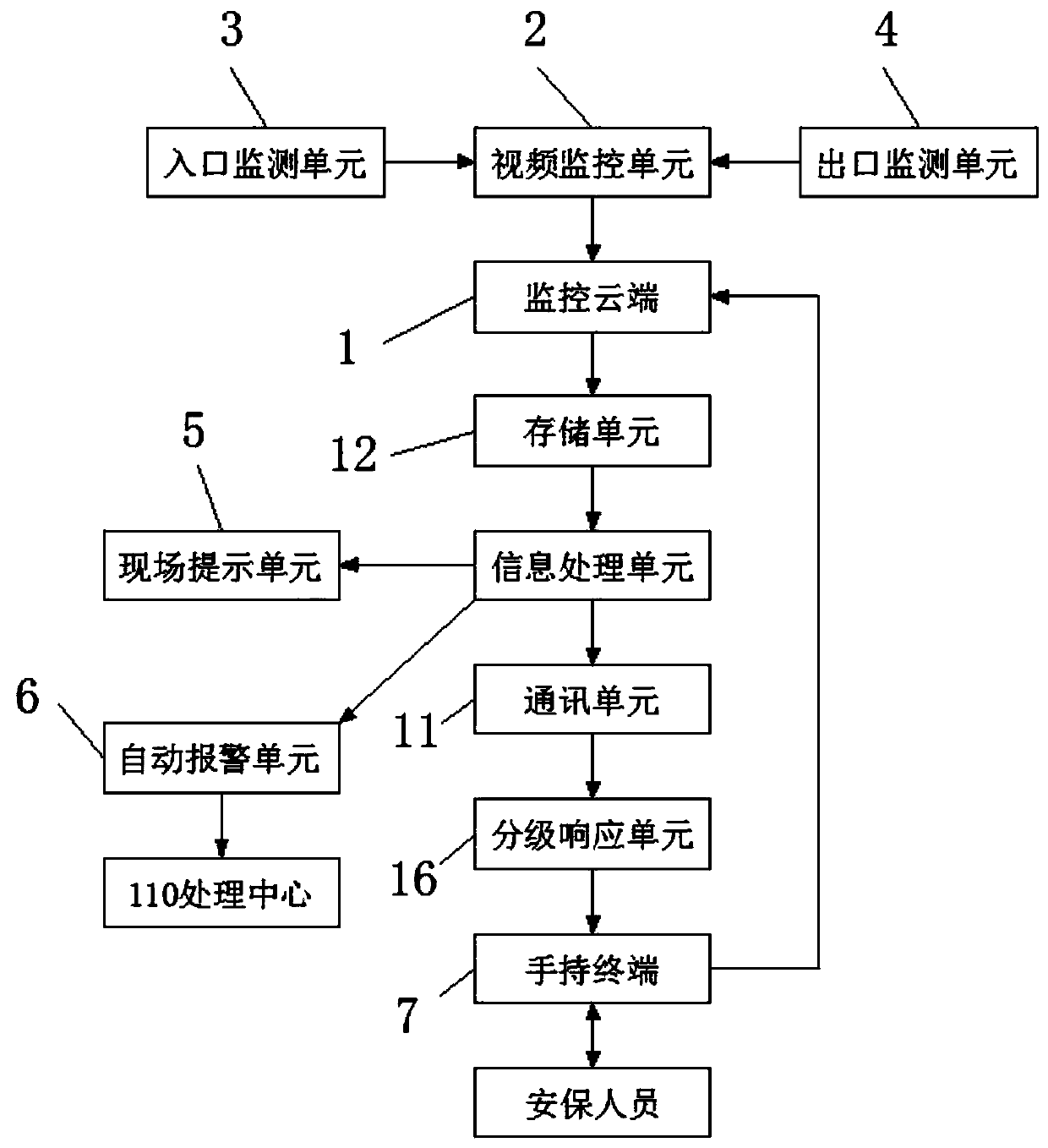
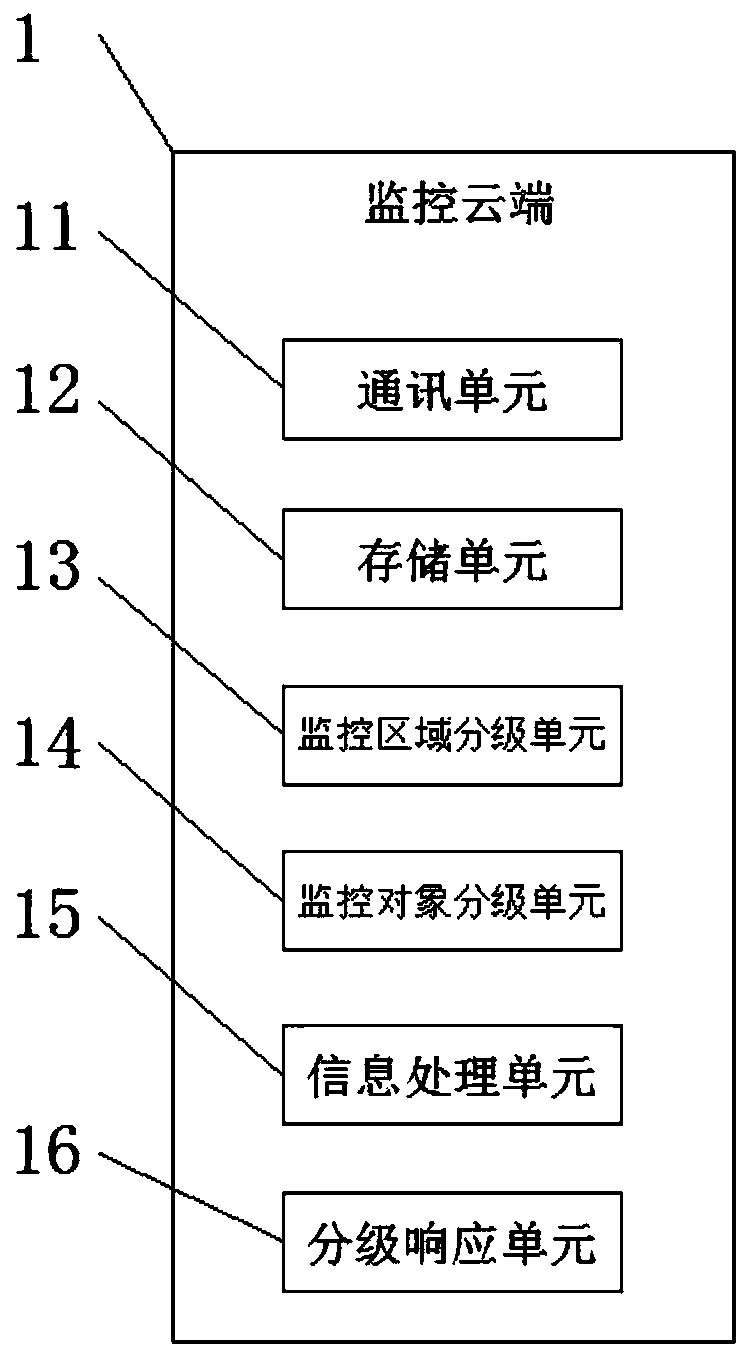
InactiveCN109671001AEasy accessImprove monitoringTelevision system detailsData processing applicationsVideo monitoringMonitoring system
The invention discloses an intelligent community safety monitoring system and a safety monitoring method. The system comprises a monitoring cloud, a video monitoring unit, an entrance monitoring unit, an outlet monitoring unit, a field prompting unit, an automatic alarm unit and a hand-held terminal. The monitoring cloud is arranged in an equipment operation room; the video monitoring unit is connected with the monitoring cloud through an electric signal transmitted in the electronic connecting line; the entrance monitoring unit is installed at an entrance of a community and is connected withthe video monitoring unit via an electric signal transmitted in the electronic connecting line; the exit monitoring unit is installed at a community exit and is connected with the video monitoring unit through an electric signal transmitted in the electronic connecting line, the on-site prompting unit is installed on the community monitoring device, and the automatic alarm unit is connected withthe monitoring cloud through an electric signal transmitted in the electronic connecting line. According to the smart community safety monitoring system and the safety monitoring method, the identities of people entering and exiting the community can be identified in time, and the community comfort level index is improved.
Owner:上海东冠通信建设有限公司
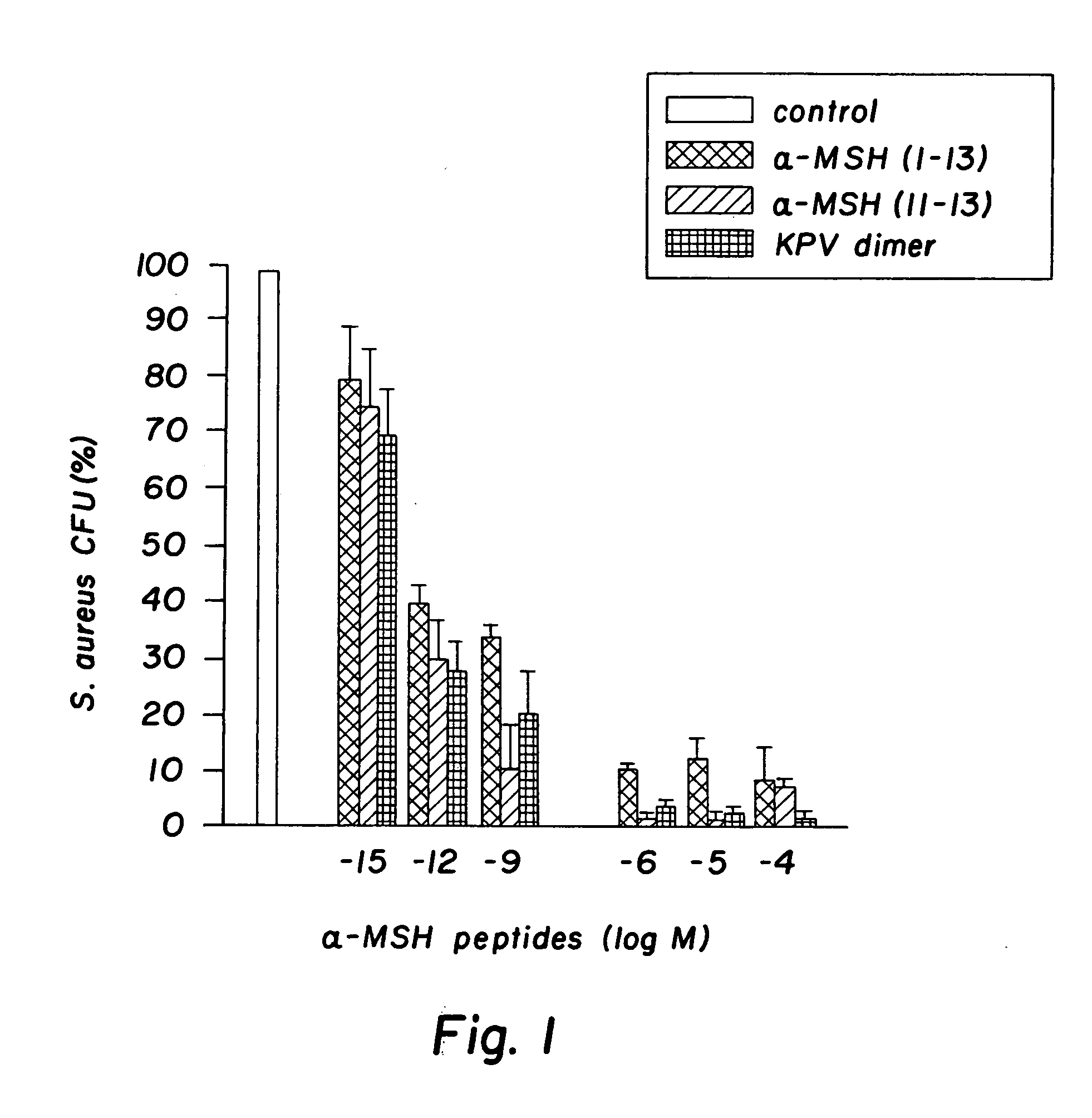
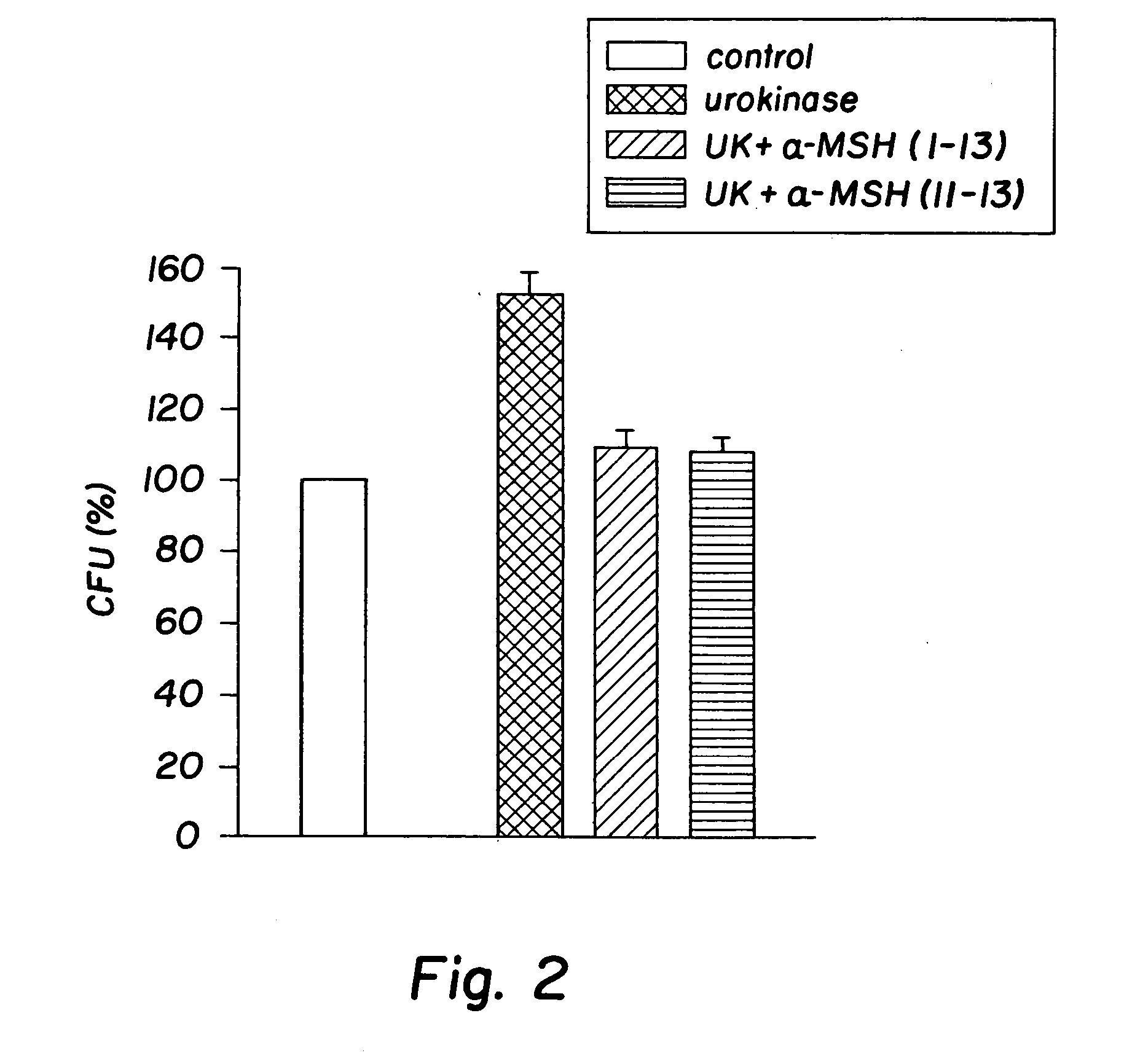
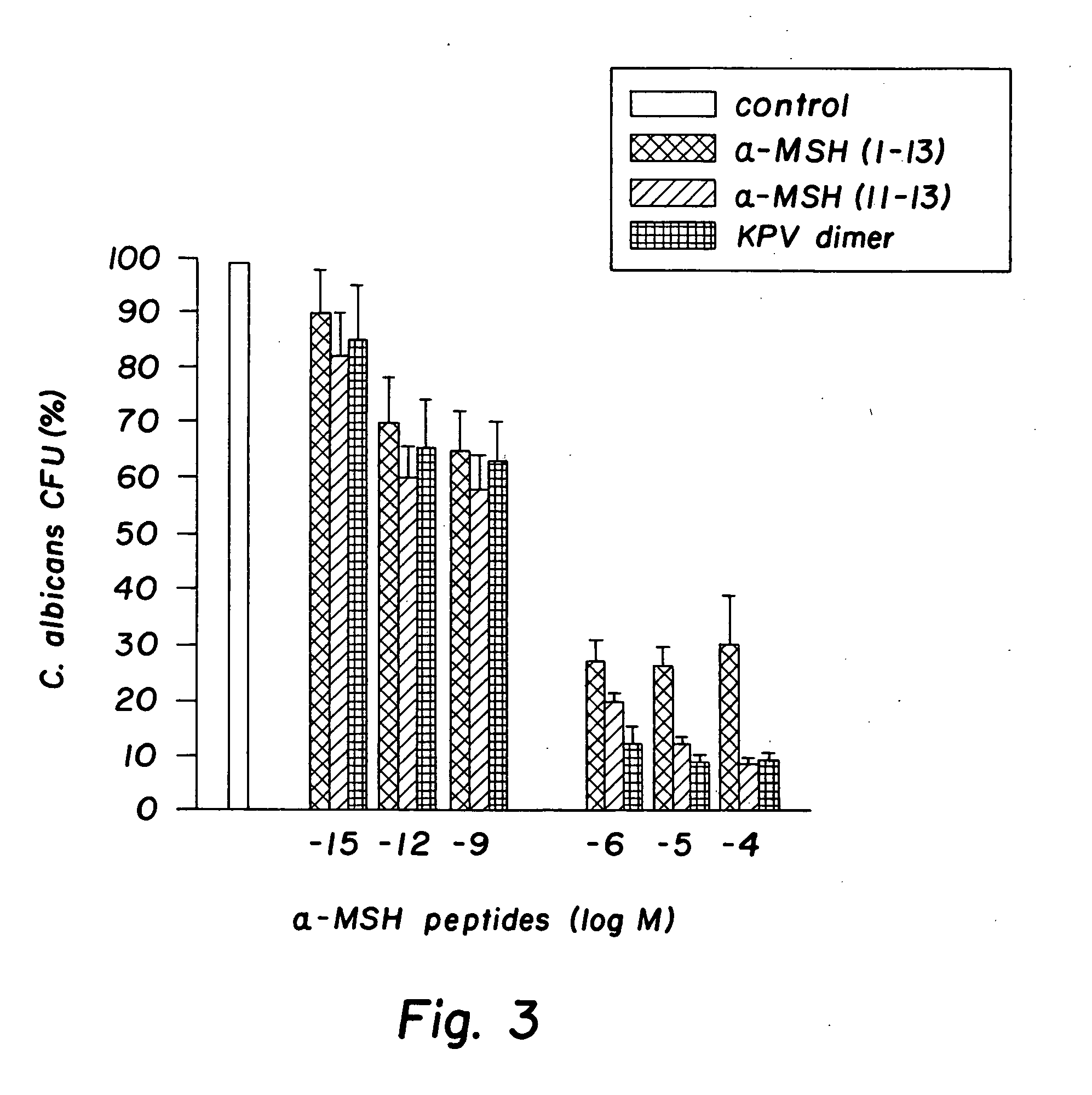
Antimicrobial amino acid sequences derived from alpha-melanocyte-stimulating hormone
InactiveUS20060111300A1Reduced viabilityAccelerate the accumulation processBiocideAntimycoticsDiseaseAmino acid
Owner:ZENGEN
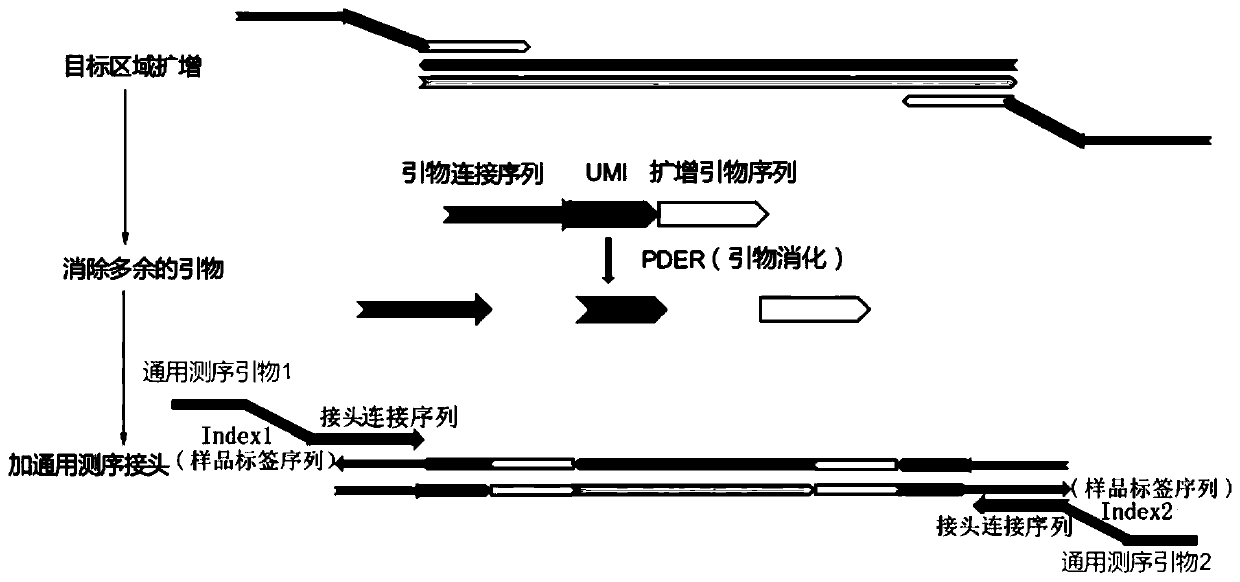
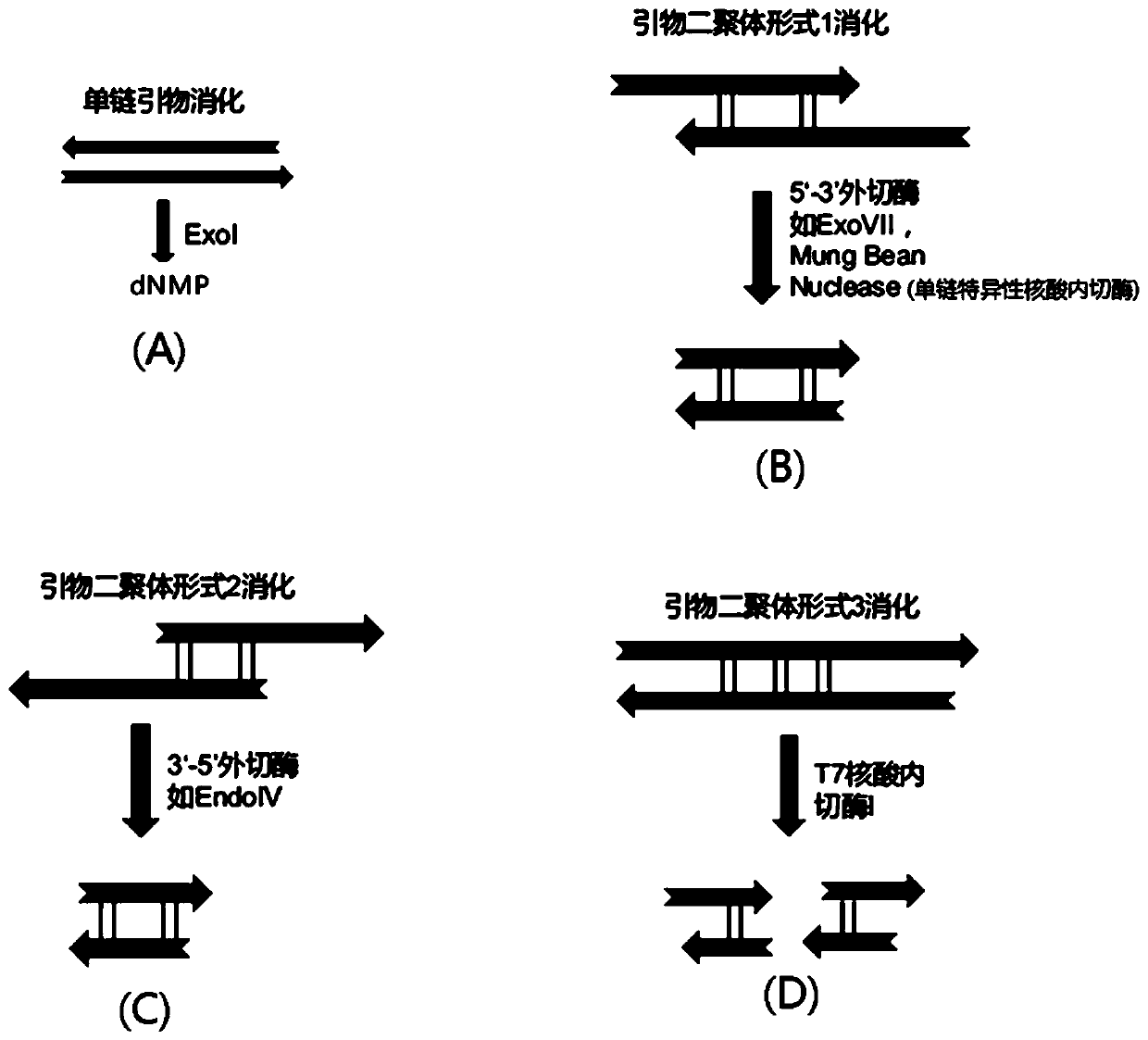
Method and kit for enriching circulating tumor DNA on basis of multiplex PCR
ActiveCN110117574AReduce lossesHigh detection sensitivityMicrobiological testing/measurementTumor/cancer cellsGene targetsCirculating tumor DNA
The invention discloses a method and kit for enriching circulating tumor DNA on the basis of multiplex PCR. The method comprises the steps of (1), designing multiple PCR primer pairs, wherein PCR primers comprise an amplifying primer sequence used for amplifying a gene target region and a primer ligation sequence, and the primer ligation sequence is used for being linked with a universal sequencing linker of a sequencing platform; (2), taking a template containing the circulating tumor DNA and making the template and the PCR primer pairs in step (1) subjected to a first PCR reaction to obtaina first PCR reaction product; (3), adding a combined enzyme into the first PCR reaction product to digest residual PCR primers; (4), adding the universal sequencing linker into the first PCR reactionproduct and carrying out a second PCR reaction to obtain a sequencing library. The method and the kit have the effect of reducing sample loss.
Owner:常州桐树生物科技有限公司
Production device, in particular a folding press and a method for operating a production device
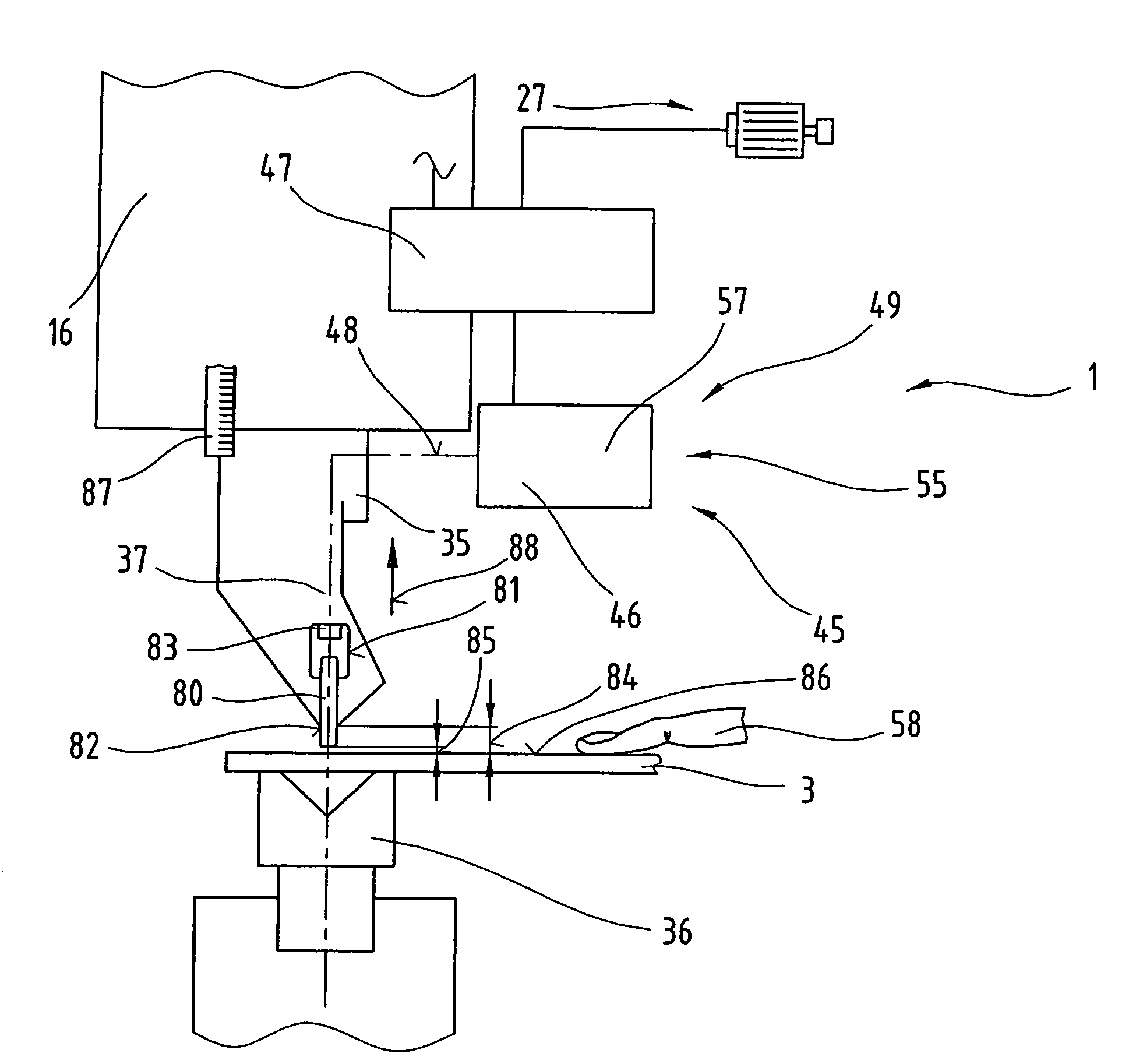
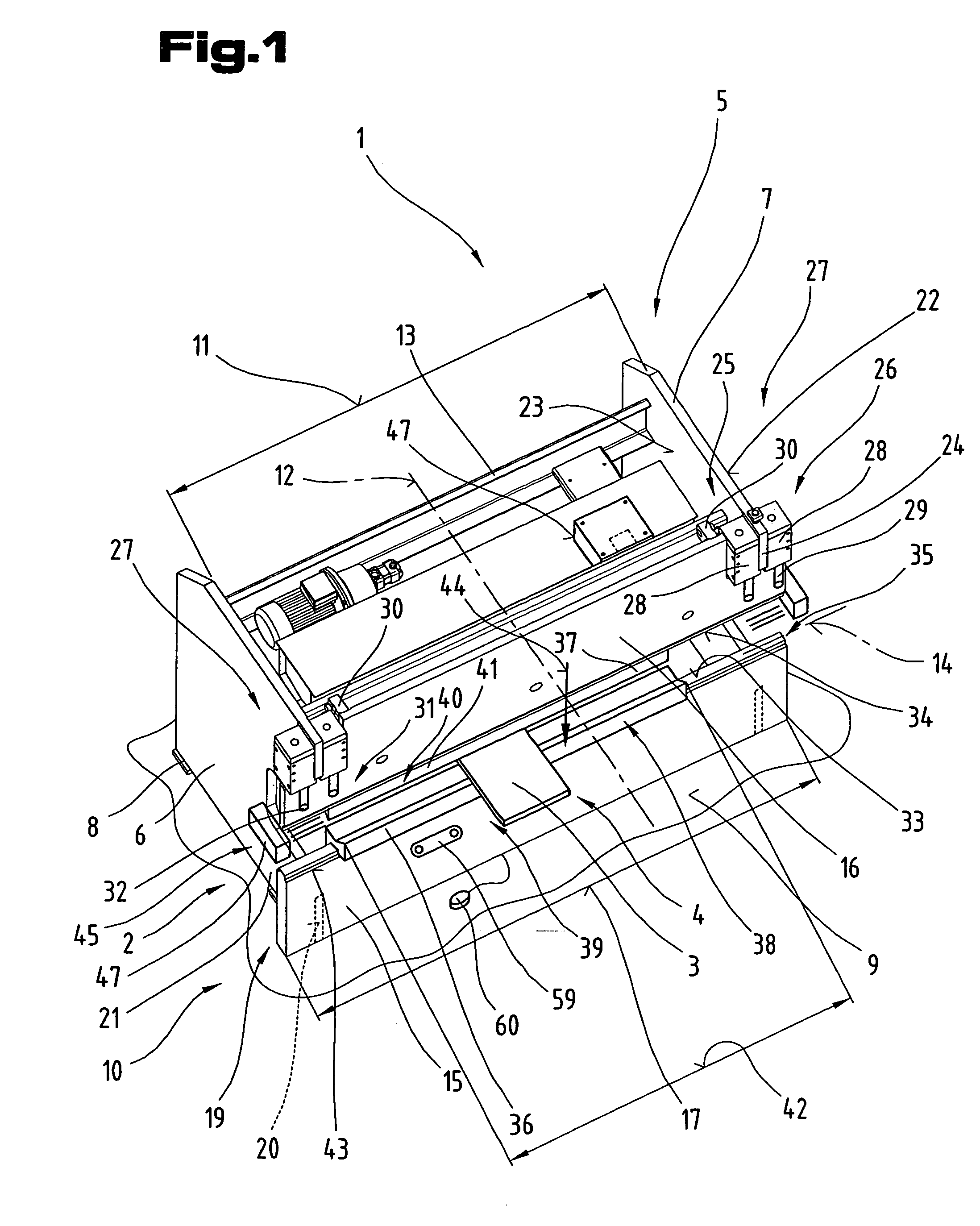
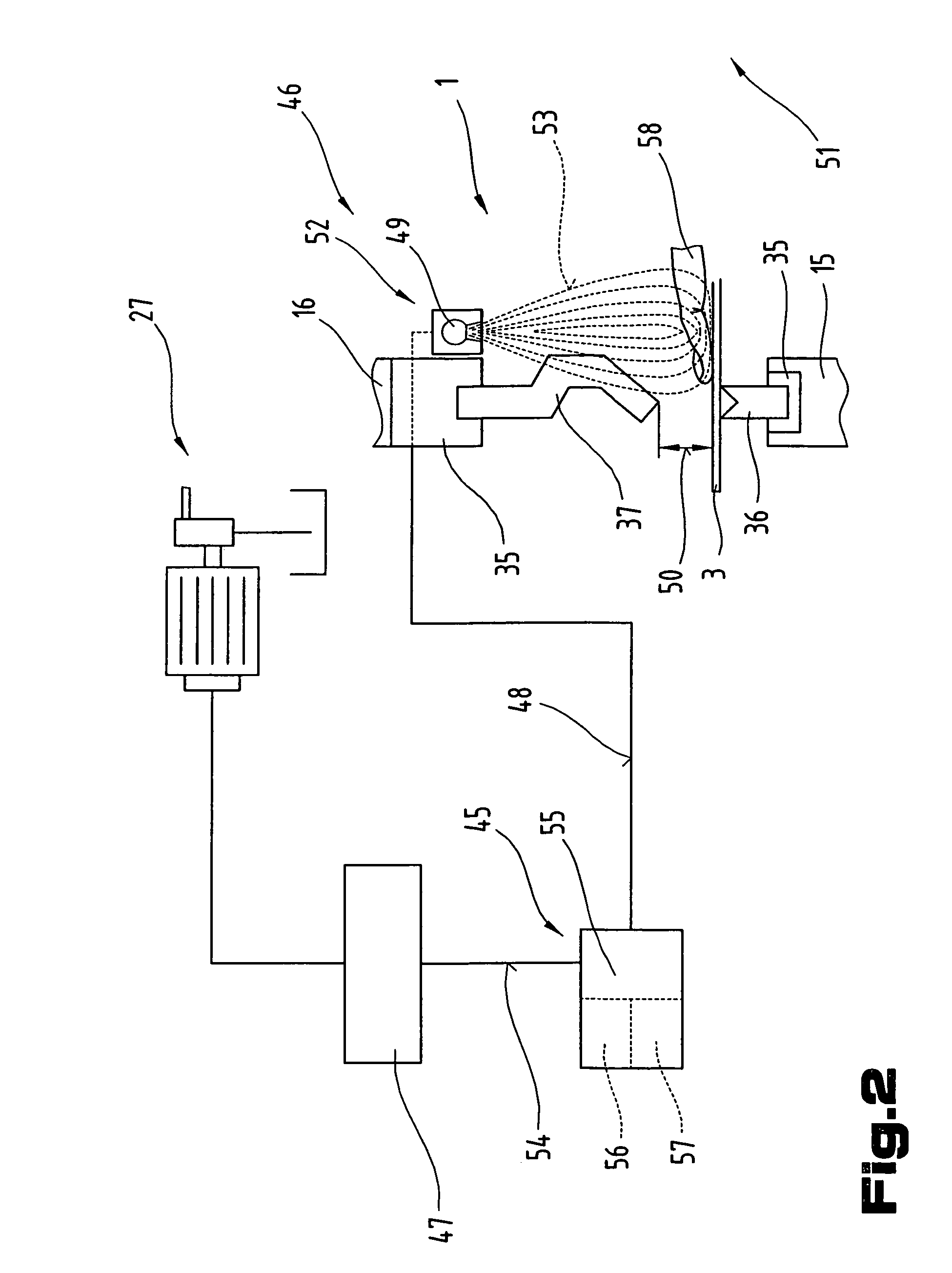
ActiveUS7281403B2Monitoring is limitedRapid responseYielding couplingSlip couplingProduction unitBiomedical engineering
The invention relates to a production unit (1), in particular a bending press, and a method of operating the latter in order to shape workpieces of sheet metal between two press beams (15, 16) equipped with bending tools (36, 37) and displaceable relative to one another by means of a drive system (27), and having a control unit (47). The control unit (47) incorporates a detection system and has at least one detection means which is connected so as to communicate with the detection system in order to transmit data and / or signals.
Owner:TRUMPF MASCHEN AUSTRIA
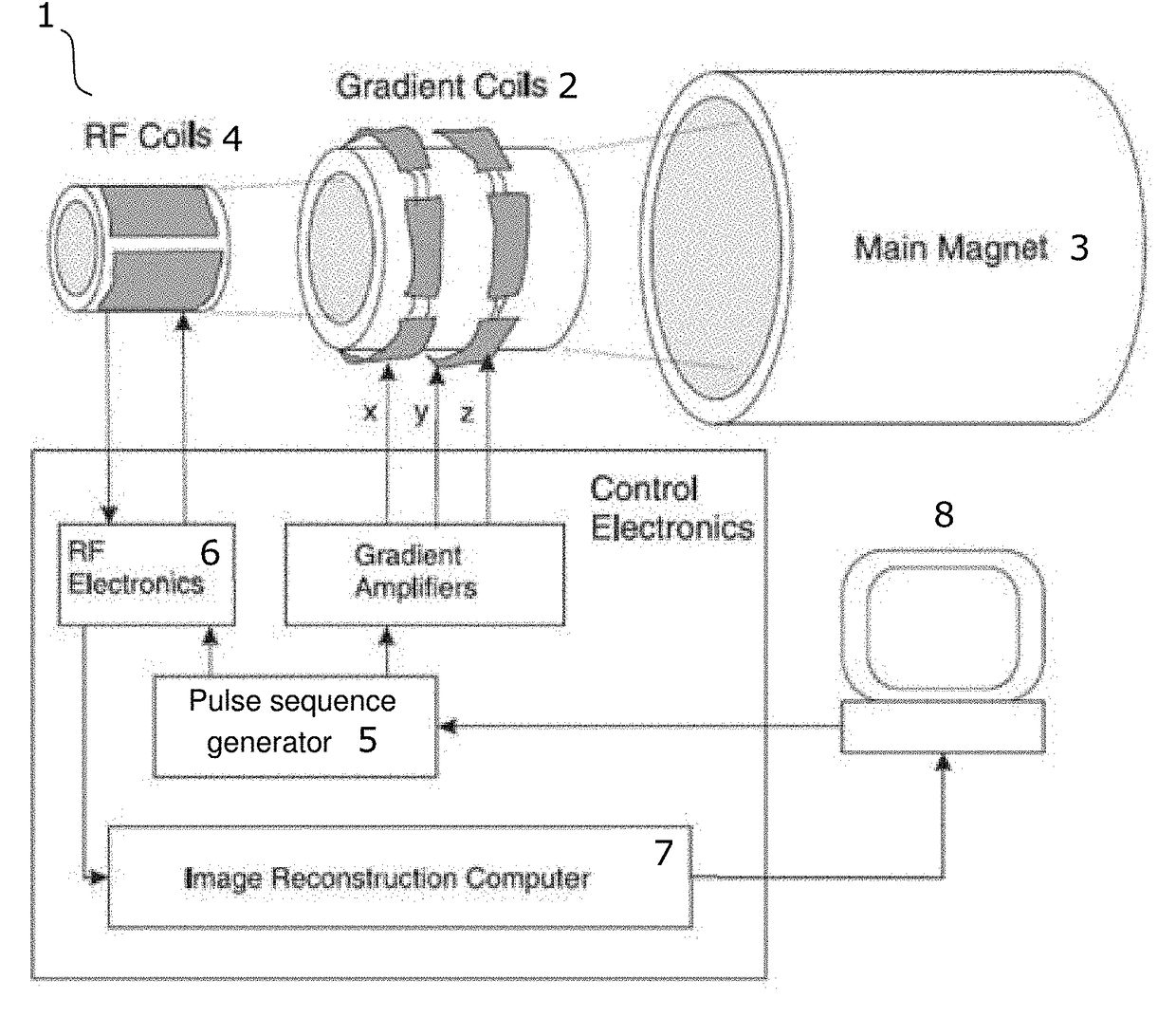
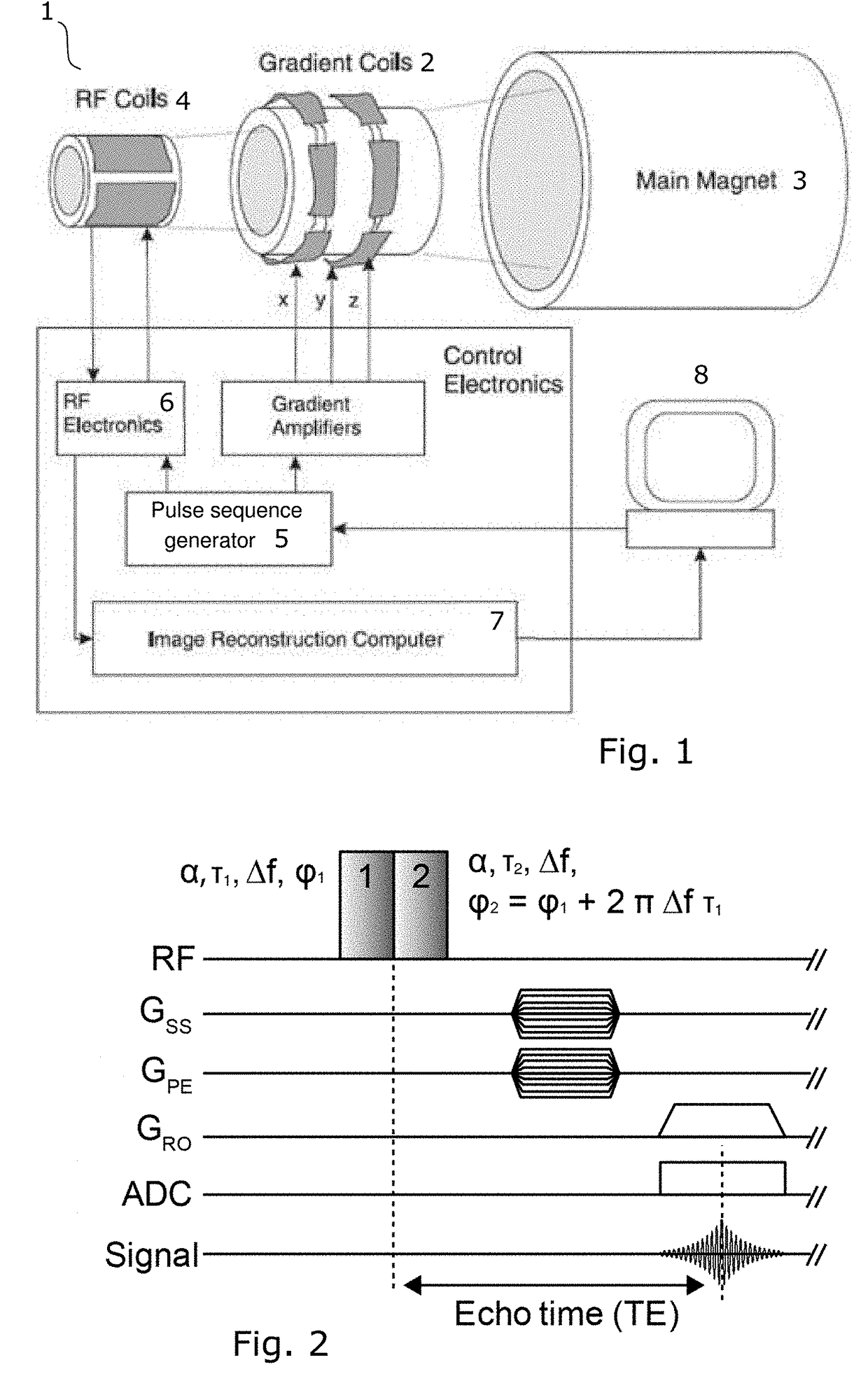
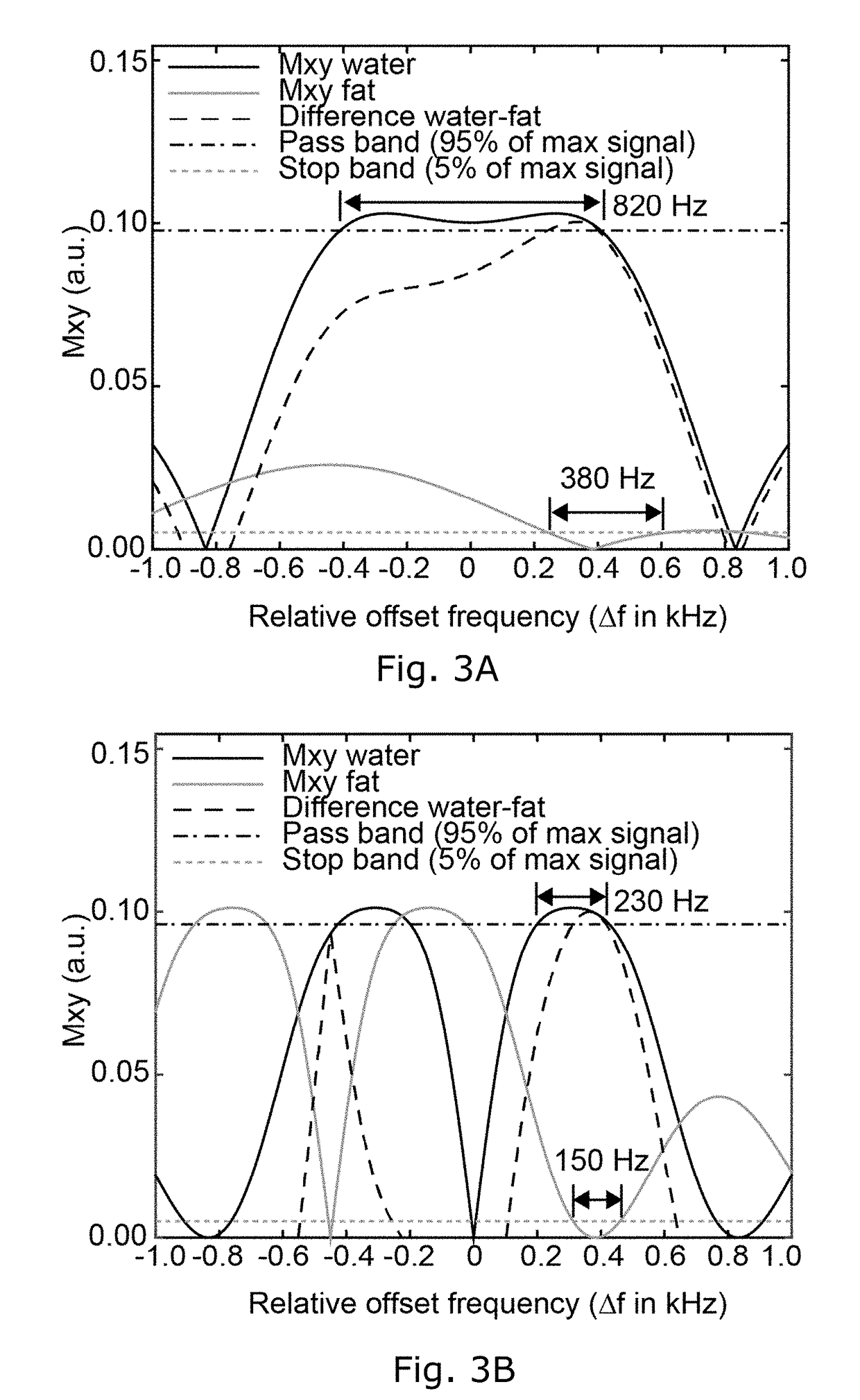
Differentiated tissue excitation in MRI
ActiveUS20170299678A1Inhibition is effectiveShort sequenceDiagnostic recording/measuringMeasurements using NMR imaging systemsFat suppressionRelative phase
Selectively exciting bulk protons in certain tissue components, e.g. water, while suppressing the excitation of others, e.g. fat, can lead to images with better contrast for desired features. The invention provides binomial, off-resonance RF excitation pulses for differentiating tissue excitation that yields a larger fat suppression that prior art water excitation methods. Proper balancing of the frequency offset and the pulse duration with a relative phase offset between the pulses leads to large-bandwidth pass- and stopbands for water and fat, respectively. The pulses can be applied with short, or even zero, interpulse delay, leading to substantial time savings in the imaging sequence.
Owner:CENT HOSPITALIER UNIV VAUDOIS C H U V
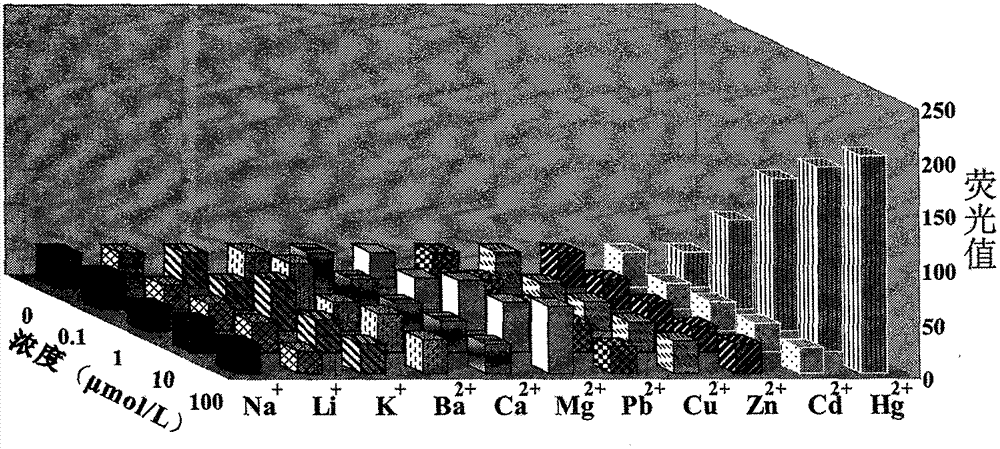
Mercury ion fluorescence detection method and kit
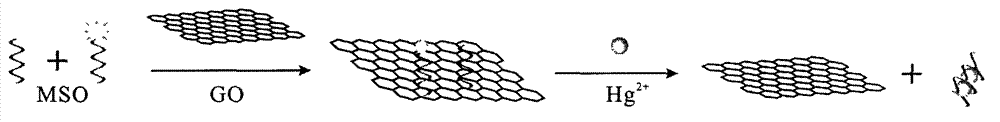
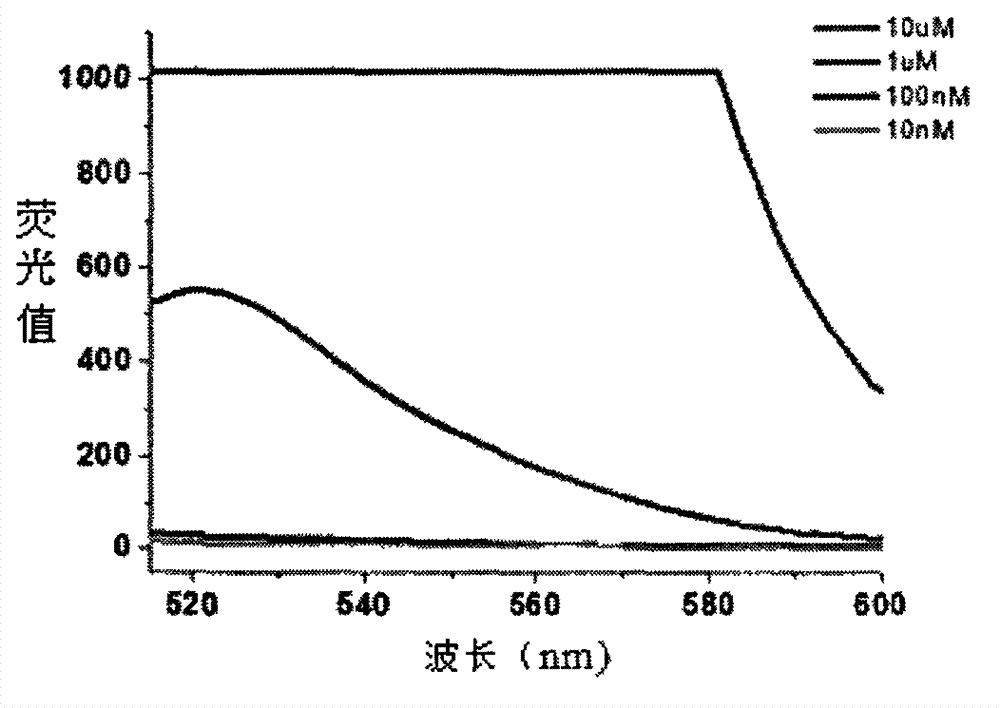
The invention relates to a mercury ion fluorescence detection method and a kit for rapid detection of mercury ions in water. Specifically, original MSO (mercury-specific oligonucleotide) is optimized and modified, fluorescence modified and non-fluorescence modified optimized MSO is adsorbed to the surface of GO (graphene oxide) by means of a non covalent bond's (pi-pi stacking) acting force, and MSO and mercury ions are utilized to form a T-Hg<2+>-T structure, so that fluorescently-labeled MSO can be released from the GO surface, and fluorescence signals can be restored. By detecting the change of fluorescence signals in a solution, quantitative detection of mercury ions in the solution can be realized. The method provided in the invention can realize convenient analysis of mercury ions without requiring any special condition. The lowest detection limit is 10pM, the sensitivity of original methods is enhanced by 18 times, and the the cost of DNA synthesis is reduced. Thus, the invention provides a simple, efficient, sensitive and cost-effective solution for monitoring mercury ion pollution in water, and the solution is easy to promote and use.
Owner:WENZHOU MEDICAL UNIV
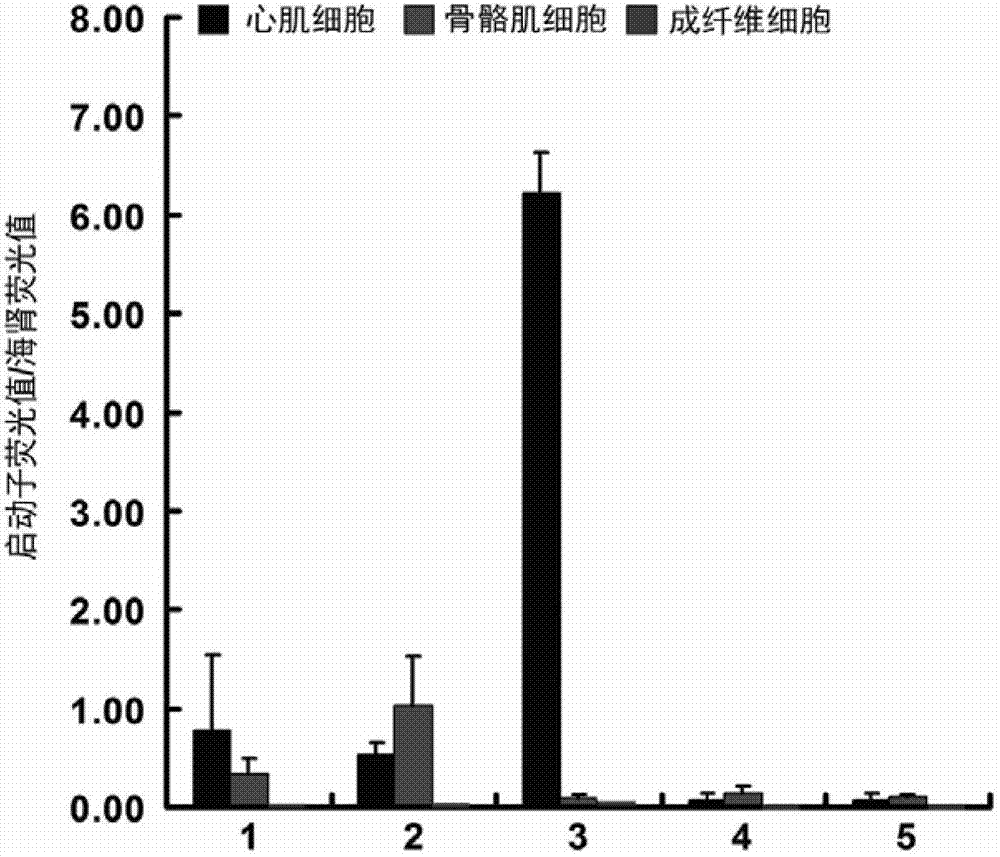
Myocardial specific promoter
InactiveCN103173451AShort sequenceGood myocardial specificityGenetic material ingredientsGene therapyCytosineVascular disease
The invention relates to a myocardial specific promoter, which is a part of a TroponinI promoter and comprises a component (TATA / MEF-2) with rich A / T, two GATA components and an area (comprising CACC box and Sp1 element) with rich cytosine; and preferably, the myocardial specific promoter is a part from the -791st site to the 67th site of a TNNI3 gene, and more preferably, the myocardial specific promoter is a part from the -234th site to the 67th site of the TNNI3 gene. The invention also relates to a nucleic-acid construction body, a carrier and a reconstitution cell comprising the promoter. The invention also relates to an application of the promotor for controlling the specific expression of target genes in myocardium and an application for preparing a medicine for preventing or treating cardiovascular disease. The promoter has the advantages that the target gene can be controlled for high expression to myocardial specificity and a new path for preventing or treating the cardiovascular disease is opened up.
Owner:JIANGSU PROVINCE HOSPITAL
Fatty-acid-modified ultra-short sequence antibacterial peptide analogue and application thereof
PendingCN110938112AShort sequenceSimple designAntibacterial agentsTripeptide ingredientsArginineAntimicrobial drug
The invention designs and synthesizes a fatty-acid-modified ultra-short-sequence antibacterial peptide analogue. The antibacterial peptide analogue is fatty-acid-modified polypeptide containing threeamino acids; arginine and tryptophan are arranged and combined in different modes to obtain an ultra-short sequence polypeptide containing three amino acids; amidation is performed on the C tail end of the ultra-short-sequence polypeptide and an N tail end of the ultra-short sequence polypeptide is modified with a C8-C18 fatty acid chain. The sequence is shorter, the design is simple, and the manufacturing cost is low. The in-vitro antibacterial experiments show that the ultra-short-sequence antibacterial peptide analogue has strong antibacterial activity on common strains, the antibacterial activity on gram-positive bacteria is superior to that on gram-negative bacteria. The ultra-short-sequence antibacterial peptide analogue has the broad application prospects in preparation of clinicalantibacterial drugs.
Owner:倪京满
Single-gene bispecific antibody capable of targeting CTLA4 and PD-1 and application thereof
InactiveCN109762068AShort sequenceExtended durationHybrid immunoglobulinsAntibody ingredientsAntibody expressionBispecific antibody
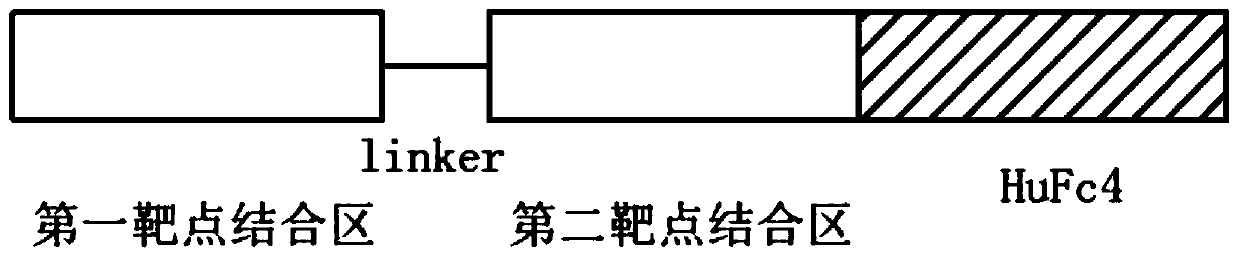
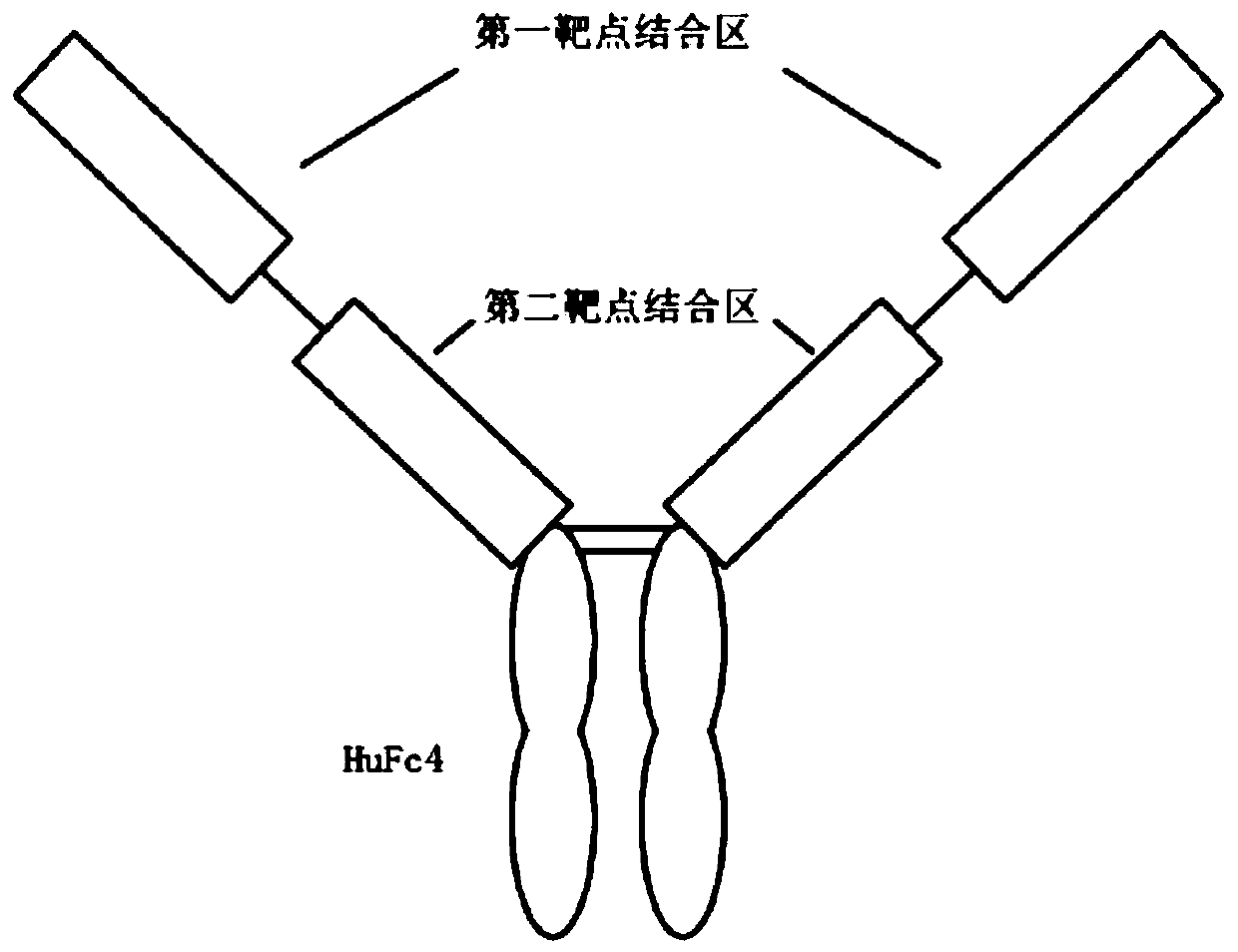
The invention relates to a single-gene bispecific antibody capable of targeting CTLA4 and PD-1. The single-gene bispecific antibody comprises a first target binding region, a second target binding region and an Fc region from an N end to a C end in sequence, wherein the first target binding region is connected with the second target binding region through a connecting fragment, and the first target binding region targets one of the CTLA4 and PD-1, the second target binding region targets the other of the CTLA4 and PD-1. The invention also relates to application of the single-gene bispecific antibody in preparation of antitumor drugs. The invention also relates to an AAV bispecific antibody expression vector. The single-gene bispecific antibody can effectively target the PD1 and CTLA4, is short in sequence and simple in structure, is suitable for construction of an AAV virus-based antibody expression vector, the antibody expression vector can be directly injected, and the maintenance time of the antibody in the body is prolonged.
Owner:Y CLONE MEDICAL SCI CO LTD
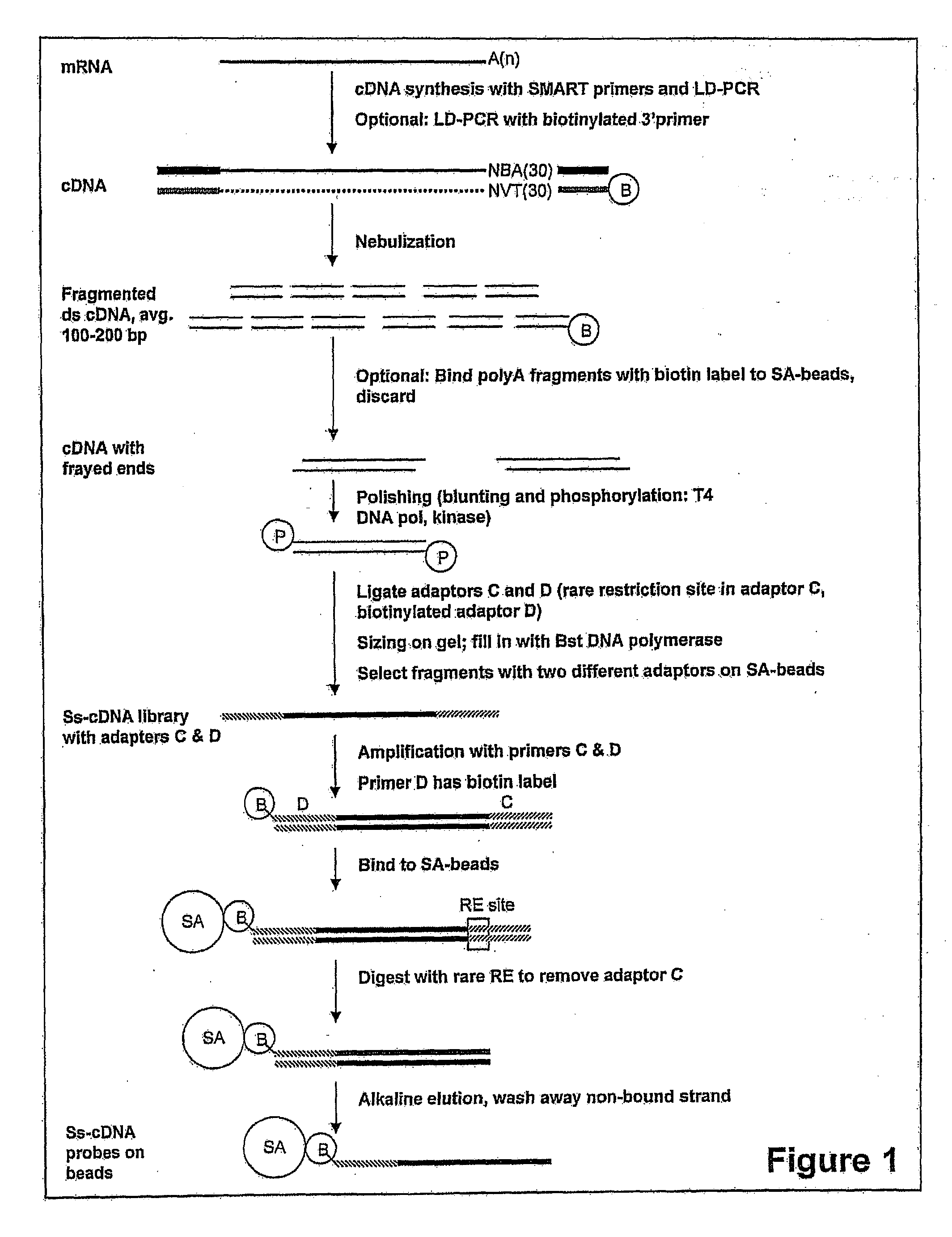
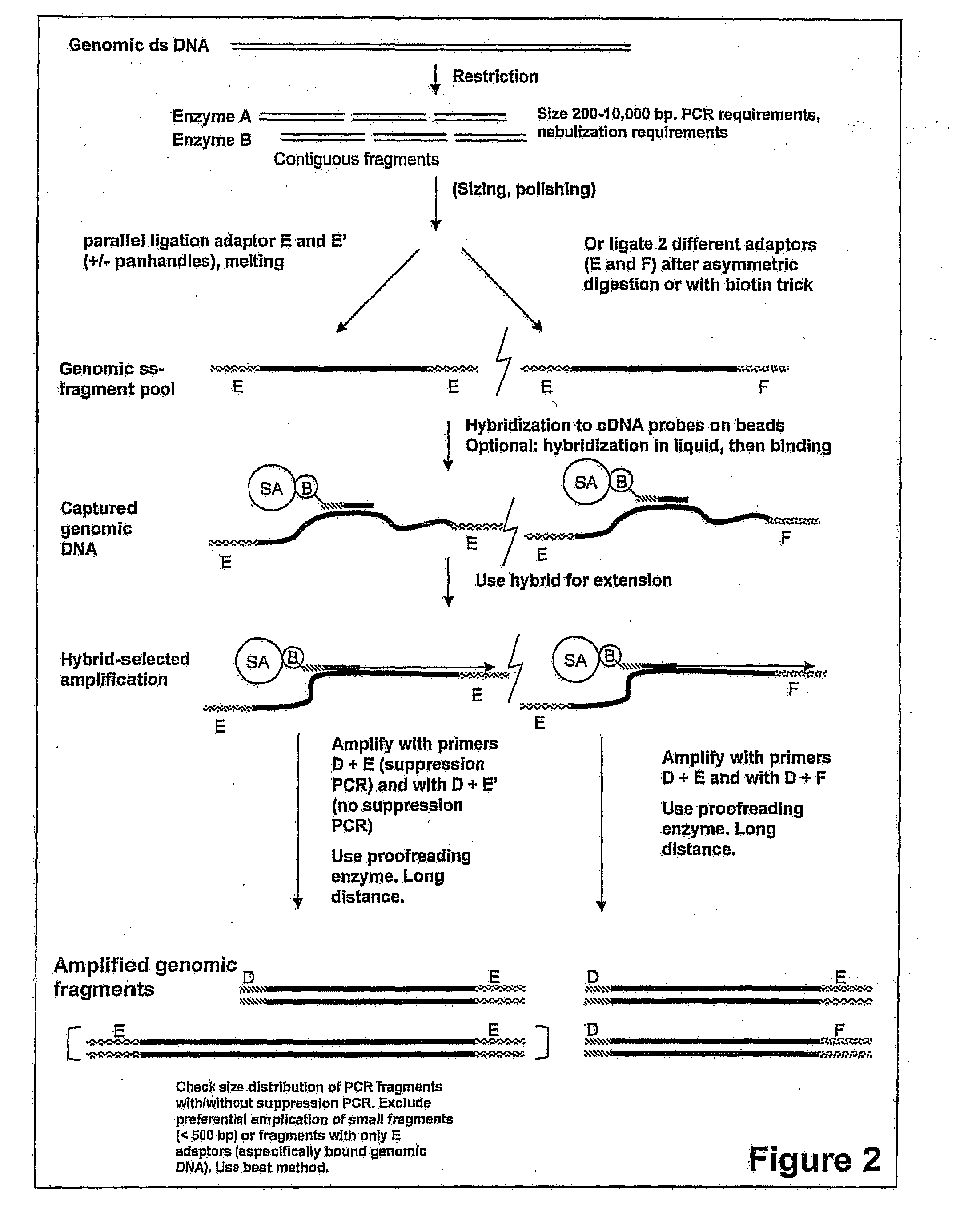
Expression-linked gene discovery
InactiveUS20110105338A1Increase volumeImprove cloning efficiencyNucleotide librariesMicrobiological testing/measurementGenomic SegmentAssociated organism
The invention relates to a method for analyzing a genomic region of an organism, comprising four major parts. The first part involves the isolation of mRNA from a selected organism that is used for the preparation of small single stranded DNA fragments with one adaptor containing an affinity label. These DNA fragments are used in part three. In the second part, genomic DNA from the same or a related organism is isolated. This genomic DNA is fragmented and ligated to adaptor molecules. In the third part, these genomic fragments are hybridized with single stranded DNA fragments from part one, and the hybrids formed in this process are used for synthesis of DNA fragments. These fragments will be used in part four which involves sequencing of these fragments using one of the available high throughput sequencing methods.
Owner:STICHTING GENETWISTER IP
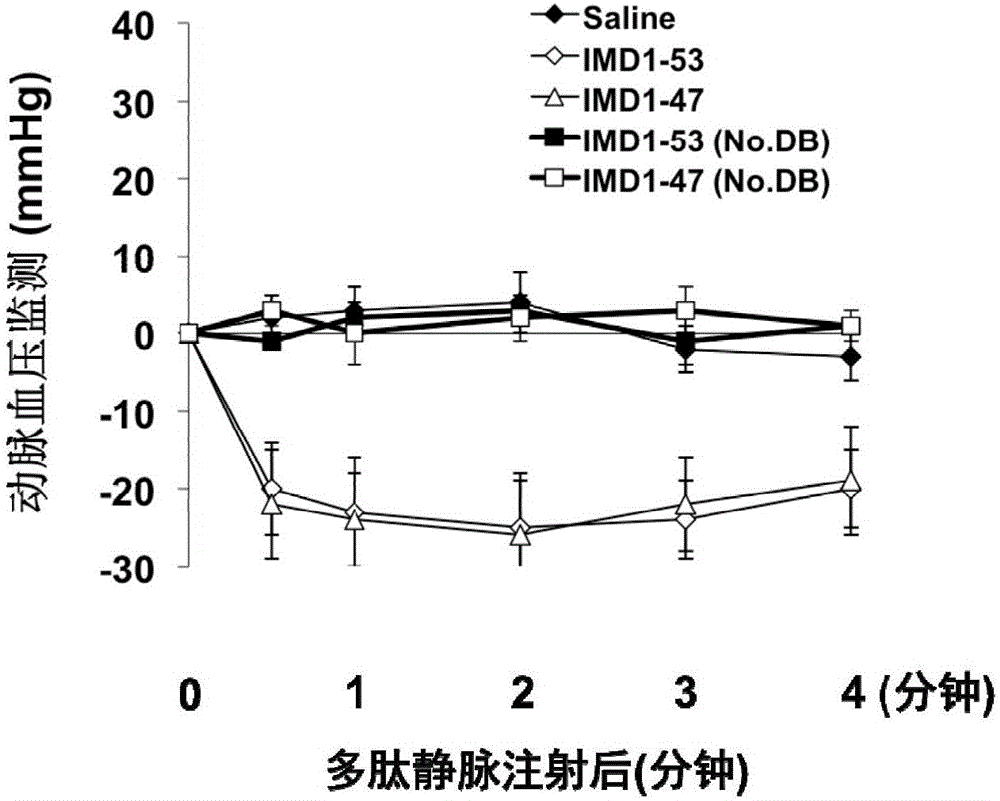
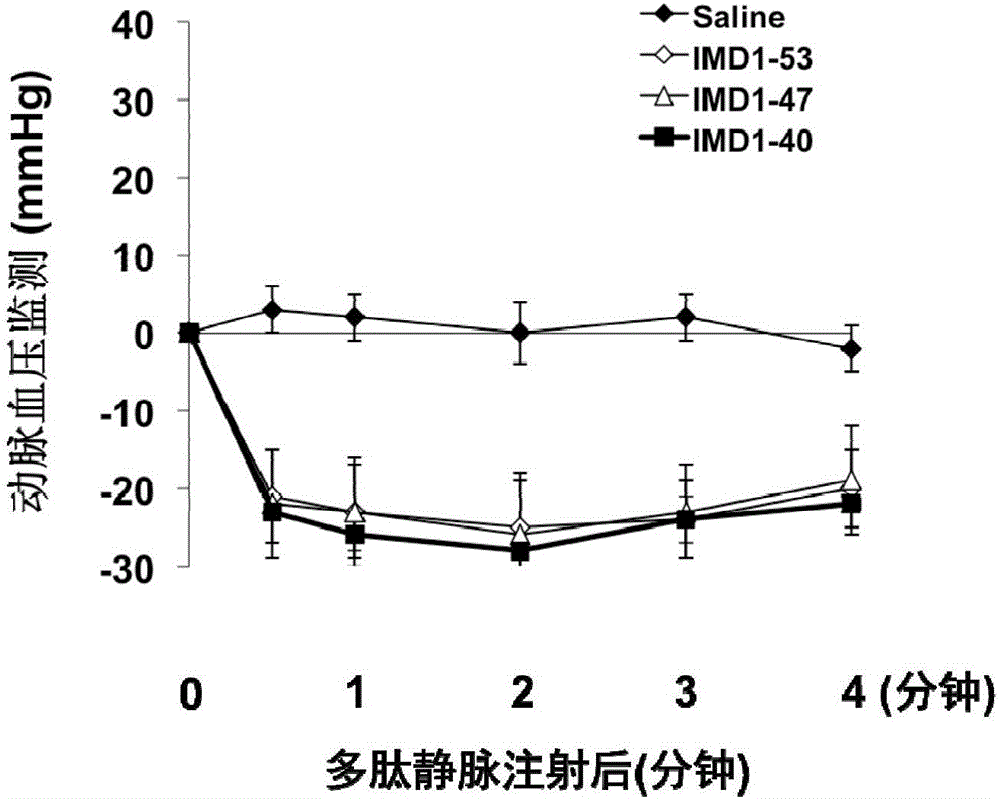
Intermedin related polypeptide and application thereof in preventing and treating sepsis
ActiveCN106749612AReduce leakageReduce edemaAntibacterial agentsPeptide/protein ingredientsVascular endotheliumAdrenomedullin 2
The invention belongs to the field of biological medicine and relates to polypeptide fragment derived from intermedin (IMD; alternate name, Adrenomedullin 2, ADM2). The polypeptide fragment is specifically mature peptide IMD108-147 with the length of 40 amino acids. Researches show that intermedin precursor peptide and three kinds of sheared manure peptides can reduce cytokine storm caused by sepsis, have a protection effect on vascular endothelial barriers, can reduce tissue edema and organic damage, can effectively reduce death rate of the sepsis. The IMD108-147 related in the invention has possibility of serving as polypeptide medicine to be applied to preventing and treating clinical sepsis and has a very good application prospect.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Actuator having at least one control element which has thermal transducer material
ActiveUS20140298794A1Fast reaction timeShort time sequenceMechanical power devicesClosed-cycle gas positive displacement engine plantElastomerMechanical energy
The invention is an actuator which includes at least one control element which has thermally activatable transducer material and which, in response to the supply or dissipation of energy, changes from a first shape state into a second shape state, and a mechanical energy store, which is functionally connected to the control element and, when the control element is in the second shape state, exerts on the control element a restoring force which returns the control element to the first shape state. The mechanical energy store includes an elastomer body, which at least in some regions is in direct physical and thermal contact with the control element, the elastomer body is connected in a spatially fixed manner to the control element in at least two spatially separated joining regions along the control element.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
Identification method of rice bacterial leaf blight resistance and application of miRNA1425 genetic locus
ActiveCN103184285AShort sequenceEasy to degradeMicrobiological testing/measurementBiotechnologyMiRNA Gene
The invention discloses an identification method of rice bacterial leaf blight resistance and an application of miRNA1425 genetic locus, and belongs to the field of biotechnology. The identification method comprises the following steps: inoculating paddy rice to be detected and infected paddy rice; separating miRNA genes of the paddy rice to be detected and the infected paddy rice; detecting the expressions of the miRNA genes; and determining whether the paddy rice to be detected is resistant to bacterial leaf blight. The invention further discloses the application of the miRNA1425 genetic locus in identification of rice bacterial leaf bright resistance. The invention uses the transcription of the miRNA1425 gene locus to identify whether paddy rice is resistant to bacterial leaf blight, and achieves success. The transcription of the miRNA1425 gene locus provided by the invention is detection based on expression level, so as to avoid misjudgment caused by bacterial leaf blight resistance gene silencing; and the identification method provided by the invention does not use the conventional DNA linkage tracer method, so as to avoid misjudgment caused by untightness of DNA linkage, improve the detection accuracy and avoid unnecessary production loss.
Owner:JIANGHAN UNIVERSITY
Combined multiple-displacement amplification and PCR in an emulsion microdroplet
ActiveUS11142791B2Easy to identifySequencing is facilitatedHeating or cooling apparatusMicrobiological testing/measurementNucleic acid amplification techniqueEmulsion droplet
The methods and systems described herein provide an improved emulsion droplet based nucleic acid amplification method, which allows nucleic acids contained in biological systems to be detected, quantitated and / or sorted based on their sequence as detected with nucleic acid amplification techniques, e.g., polymerase chain reaction (PCR). The nucleic acids can be free floating or contained within living or nonliving structures, including particles, viruses, and cells. The nucleic acids can include, e.g., DNA or RNA.
Owner:RGT UNIV OF CALIFORNIA
MSO/GO (Mercury-Specific Oligonucleotide/Graphene Oxide) based water environment mercury ion detecting method and kit
InactiveCN103173540AHigh sensitivityLow cross-reactivityMicrobiological testing/measurementFluorescence/phosphorescenceFluorescencePollution
The invention relates to an MSO / GO (Mercury-Specific Oligonucleotide / Graphene Oxide) based mercury ion detecting method and a kit for the rapid detection of mercury ion in water. According to the invention, fluorescence-modified and not-fluorescence-modified optimized MSO is adsorbed to the surface of GO by utilizing the acting force of non-covalent bonds (pi-pi superposition); a structure of T-Hg2+-T is formed by the MSO and the mercury ion to ensure that the fluorescently-labeled MSO is released from the surface of the GO and the fluorescence signal is recovered; and the quantitative determination of the mercury ion in solution is realized through detecting the changes of the fluorescence signal in the solution. The method disclosed by the invention has the advantages that the convenient and fast analysis of the mercury ion can be realized without any special condition; the lowest detection limit is 10pM; the sensitivity is greatly increased; the DNA (DeoxyriboNucleic Acid) synthetizing cost is reduced; a solution which is simple, convenient, efficient, sensitive and cost performance is provided for the monitoring of the pollution of the mercury ion in the water; and the method is easy to popularize.
Owner:WENZHOU MEDICAL UNIV
Macrobrachium rosenbergii estrogen-related receptor gene ERR and application thereof
ActiveCN105624169AHigh activityConvenient sourceReceptors for hormonesFermentationPerturbateurs endocriniensNucleotide
The invention discloses a macrobrachium rosenbergii estrogen-related receptor gene ERR. The nucleotide sequence of the gene ERR is shown as SEQ ID NO:1, and the amino acid sequence of the gene ERR is shown as SEQ ID NO:2. The invention further discloses application of the recombined macrobrachium rosenbergii estrogen-related receptor gene ERR to environmental internal-secretion interfering-substance detection and pollution assessment, and macrobrachium rosenbergii ERR protein has important application value in the aspects of natural water environment internal-secretion interfering-substance (like NP) detection and pollution assessment.
Owner:海南永贺生物科技有限公司
Cardiac troponin I specific nucleic acid aptamer and application thereof
ActiveCN111662909AHigh affinityQuick checkDisease diagnosisBiological testingAptamerProtein detection
The invention provides a single-stranded DNA aptamer specifically binding to cardiac troponin I (hereinafter referred to as cTnI protein). The single-stranded DNA aptamer comprises a nucleotide sequence as shown in any one of SEQ ID Nos.1-3. The nucleic acid aptamer provided by the invention can also be various similar sequences with high homology or derivatives obtained from the sequences provided by the invention. The nucleic acid aptamer provided by the invention has stronger binding capacity with cardiac troponin I and has a shorter sequence, and also has the advantages of small molecularweight, easiness in synthesis, short production time, lower synthesis cost and the like. The invention also provides an application of the single-stranded DNA aptamer. The single-stranded DNA aptamercan be used for cTnI protein purification or cTnI protein detection independently or in combination. The invention also provides a kit for detecting the cTnI protein, and the kit comprises the single-stranded DNA aptamer provided by the invention.
Owner:UNIV OF SCI & TECH OF CHINA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com