Fatty-acid-modified ultra-short sequence antibacterial peptide analogue and application thereof
A fatty acid and antimicrobial peptide technology, applied in the field of biochemistry, can solve the problems of high manufacturing cost and complex design, and achieve the effect of low manufacturing cost, simple design and enhanced antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
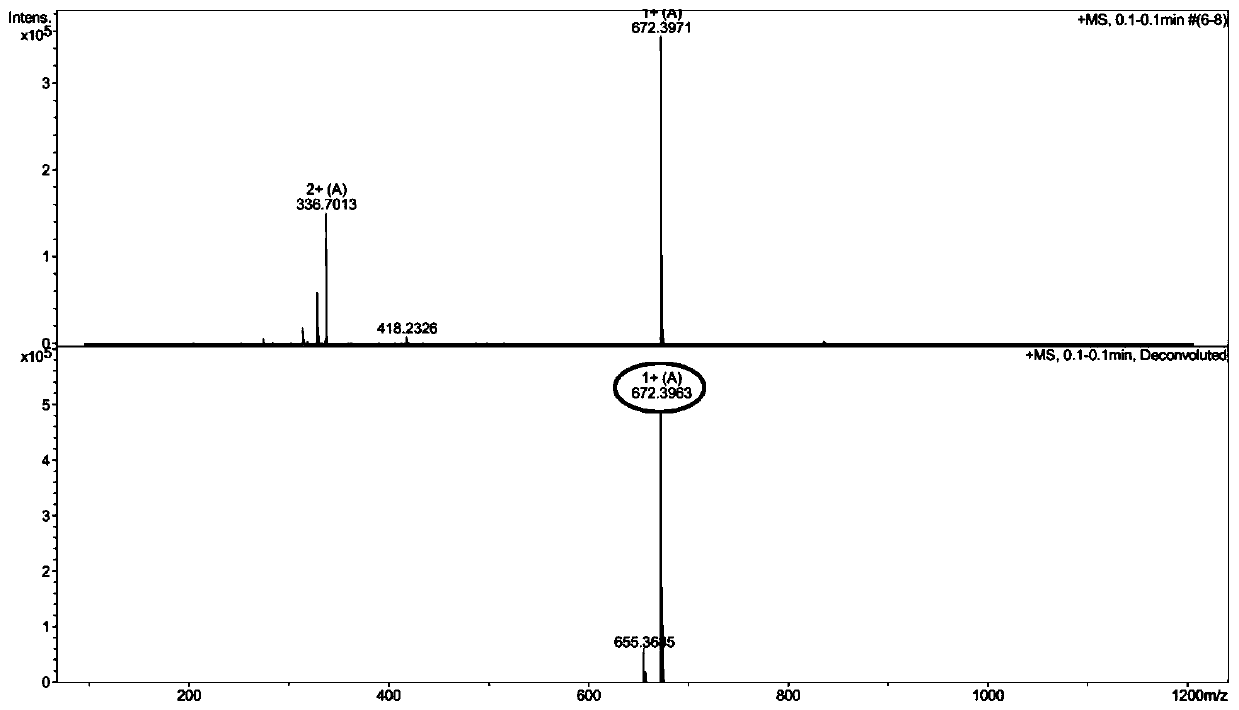
[0042] Example 1: C 8 Synthesis of -R1 and its antibacterial activity in vitro
[0043] (1)C 8 -Synthesis of R1
[0044] ①Activation and pretreatment of resin
[0045] Accurately weigh 0.58g of MBHA resin (substitution value 0.44mmol / g) and place it in a synthesizer. After swelling with DCM solution for 30min, the ninhydrin colorimetry test shows that the resin is colorless and transparent, indicating that the resin is normal.
[0046] ② Synthesis of Fmoc-R1-resin
[0047] The normal MBHA resin of the above-mentioned inspection was sloughed off the Fmoc protecting group through the DMF solution containing 20% piperidine by volume fraction, and the ninhydrin chromogenic method was tested, and the resin was blue-purple, indicating that the Fmoc protecting group had been removed; the Fmoc-Trp ( Boc)-OH (390mg), HOBT (103mg), HBTU (285mg), and DIEA (0.25mL) were dissolved in about 10mL DMF and mixed evenly, added to the synthesizer and mixed with the above-mentioned MBHA res...
Embodiment 2
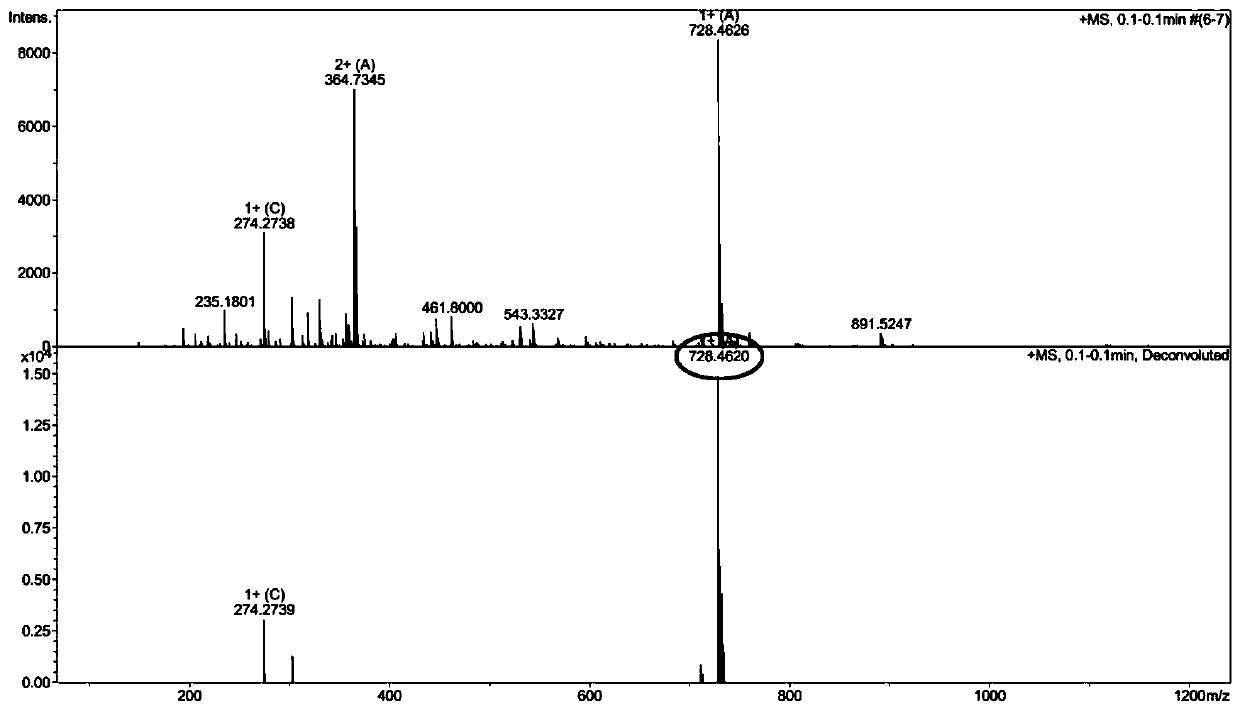
[0057] Example 2: C 12 Synthesis of -R1 and its antibacterial activity in vitro
[0058] (1)C 12 -Synthesis of R1
[0059] ①Activation and pretreatment of resin
[0060] With embodiment 1.
[0061] ② Synthesis of Fmoc-R1-resin
[0062] With embodiment 1.
[0063] ③C 12 -Synthesis of R1-resin
[0064] The Fmoc-R1-resin obtained above was also removed with the DMF solution containing 20% piperidine to remove the terminal Fmoc protecting group, and dodecanoic acid (300mg), HOBT (103mg), HBTU (285mg), DIEA (0.25mL ) was dissolved in about 10mL DMF and mixed evenly, and mixed with the above-mentioned R1-resin resin from which the Fmoc protecting group was removed, and the condensation reaction was carried out for 1.5h; the ninhydrin color method was used to test that the resin was colorless, indicating that the condensation reaction was complete, and C was obtained. 12 -R1-resin.
[0065] ④ Peptide cleavage
[0066] With embodiment 1.
[0067] ⑤ Peptide purification
...
Embodiment 3
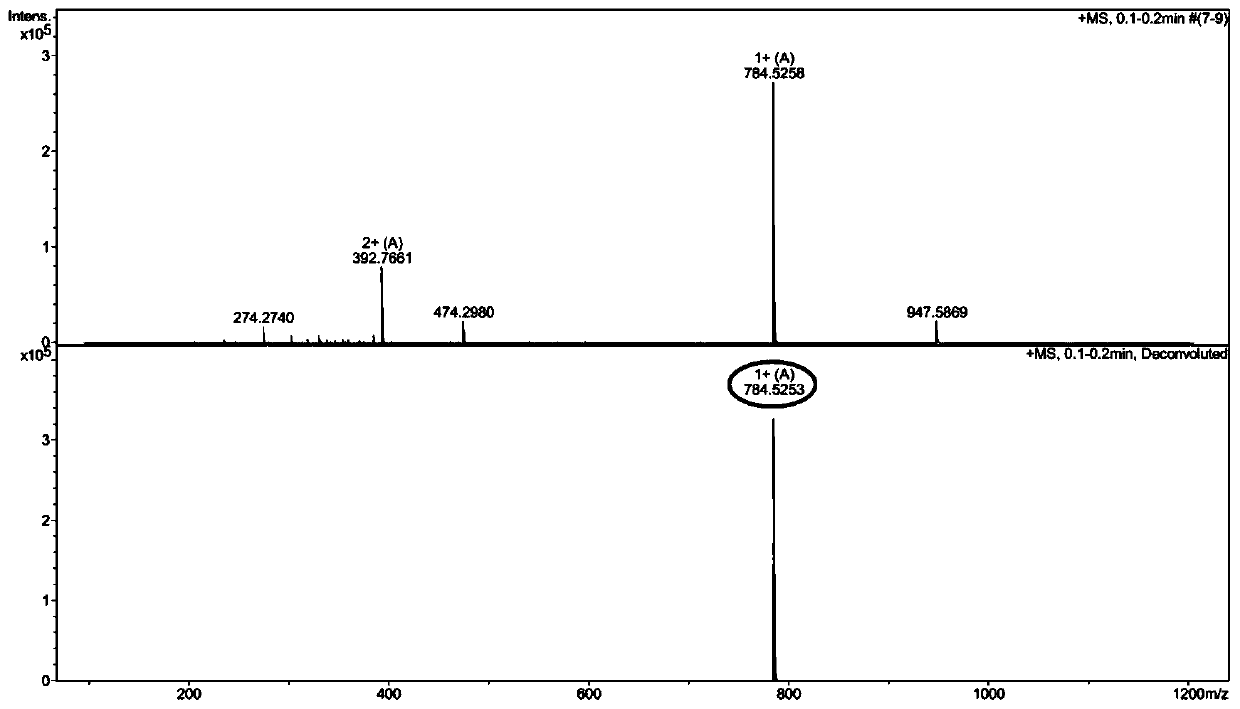
[0072] Example 3: C 16 Synthesis of -R1 and its antibacterial activity in vitro
[0073] (1)C 16 -Synthesis of R1
[0074] ①Activation and pretreatment of resin
[0075] With embodiment 1.
[0076] ② Synthesis of Fmoc-R1-resin
[0077] With embodiment 1.
[0078] ③C 16 -Synthesis of R1-resin
[0079] The Fmoc-R1-resin obtained above was also removed with the DMF solution containing 20% piperidine to remove the terminal Fmoc protecting group, hexadecanoic acid (384mg), HOBT (103mg), HBTU (285mg), DIEA (0.25mL ) was dissolved in about 10mL DMF and mixed evenly, and mixed with the above-mentioned R1-resin resin from which the Fmoc protecting group was removed, and the condensation reaction was carried out for 1.5h; the ninhydrin color method was used to test that the resin was colorless, indicating that the condensation reaction was complete, and C was obtained. 16 -R1-resin.
[0080] ④ Peptide cleavage
[0081] With embodiment 1.
[0082] ⑤ Peptide purification
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com