Cellular models of and therapies for ocular diseases
An eye disease and cell technology, applied in the cell model of eye disease and the field of therapy for eye disease, can solve the problem of lack of good diagnosis of BCD
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
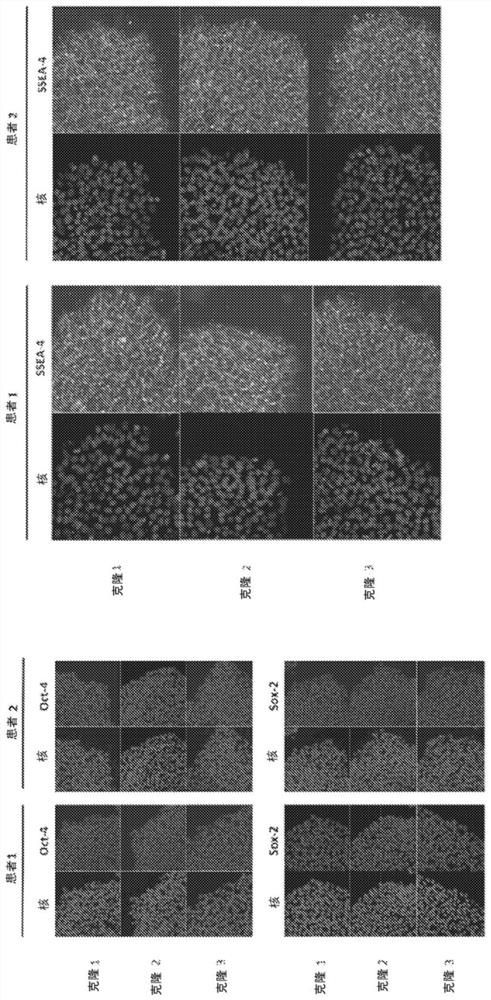
[0524] Example 1 - Generation and Characterization of Induced Pluripotent Stem Cells (iPSCs) Derived from BCD Patients
[0525] In this study, iPSCs were generated from BCD patients using an integration-free method. Traditional techniques for iPSC reprogramming (eg, lentivirus, retrovirus) integrate into the genome of the target cell. The resulting iPSCs and cells differentiated from those iPSCs will contain exogenous DNA and may be unsafe and potentially problematic for use in cell therapy and drug discovery applications. Furthermore, integration may occur in critical regions of the genome, causing problems in unrelated developmental processes. Compared to traditional reprogramming methods, integration-free reprogramming methods generate iPSCs without detectable vectors or transgenes, making them more suitable for cell therapy and drug discovery applications.
[0526] In this study, iPSCs were generated from BCD patients using two different integration-free reprogramming ...
Embodiment 2
[0548] Example 2 - Differentiation of iPSCs from BCD patients into retinal pigment epithelial (RPE) cells
[0549]The iPSC differentiation of all iPSC lines from BCD patients and healthy controls started from passage 3 to passage 6. For differentiation, iPS colonies were grown to confluence for the first 14 days in differentiation medium in 6-well dishes (Costar, Corning, Corning, NY) pretreated with Matrigel (CORNING, 356230) diluted 1:50 , the differentiation medium consisted of knockout (KO) DMEM (Thermo Fisher Scientific, 10829018), 15% KO serum substitute (Thermo Fisher Scientific, 10829028), 1% non-essential amino acids (Thermo Fisher Scientific, 11140050), 2 mmol / L gluten Aminoamide (Thermo Fisher Scientific, 35050061), 50 U / ml penicillin-streptomycin (ThermoFisher Scientific, 10378016) and 10 mmol / L nicotinamide (Sigma-Aldrich, N0636). During the 15th to 28th day of differentiation, the differentiation medium was supplemented with 100 ng / ml human activin-A (PeproTec...
Embodiment 3
[0552] Biochemical analysis, cell viability analysis and other analysis of embodiment 3-BCD cell model and CYP4V2 function research study
[0553] Lipid Analysis:
[0554] Previous studies on the function of BCD and CYP4V2 enzymes have focused on fatty acids. In this study, more lipid assays including not only fatty acids but also ceramide (Cer), sphingomyelin, (SM) and sphingosine and dihydrosphingosine (SOSA).
[0555] The biochemical analysis of free fatty acid (FFA), ceramide (Cer), sphingomyelin (SM), sphingosine and dihydrosphingosine (SOSA) was carried out at Columbia University (New York, NY). , USA) based on its relevant analysis and protocols.
[0556] Free fatty acids (FFA), ceramides, sphingosines and dihydrosphingosines were extracted by using chloroform:methanol. Briefly, approximately 1 million iPS-RPE cells were homogenized in 150 µL of water. Mix 100 μL of homogenate with 3 mL of chloroform:methanol (v:v=2:1) containing internal standard (palmitic a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com