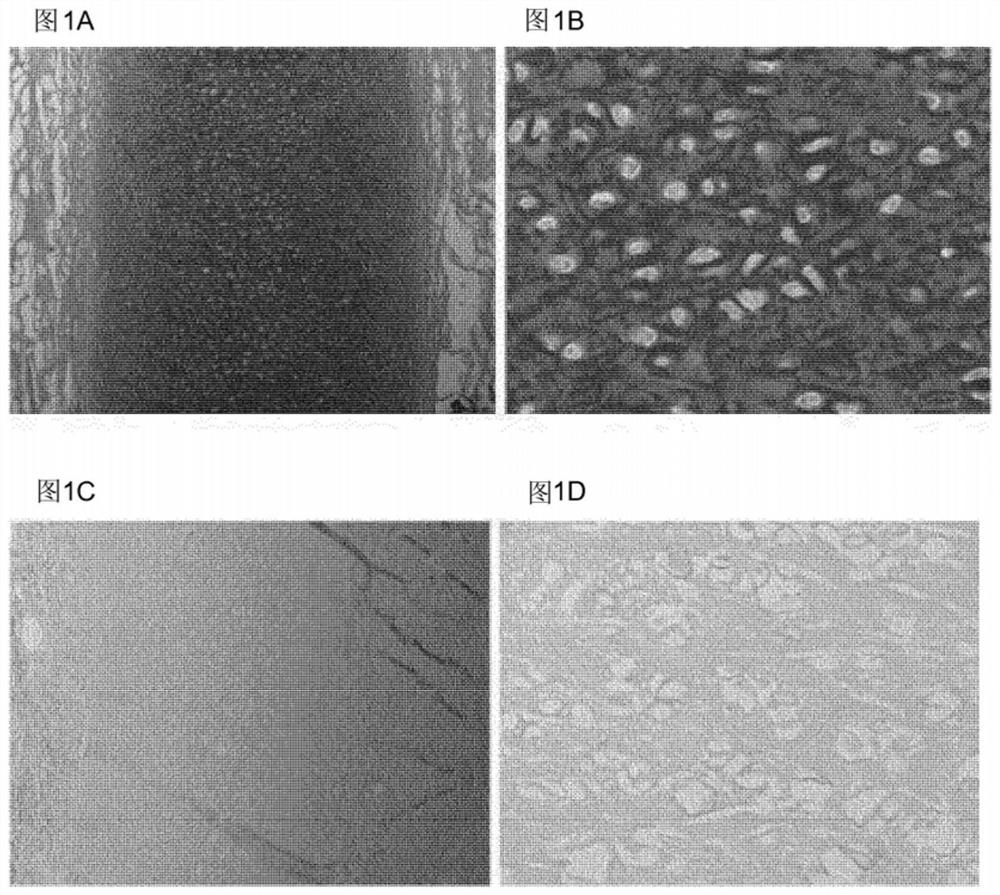
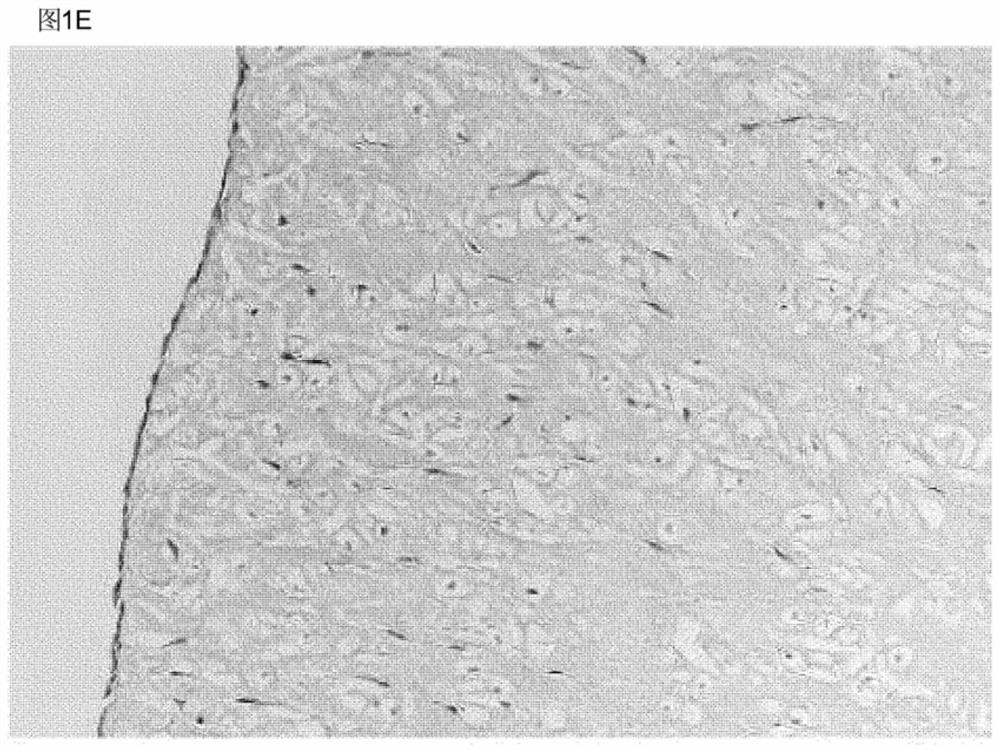
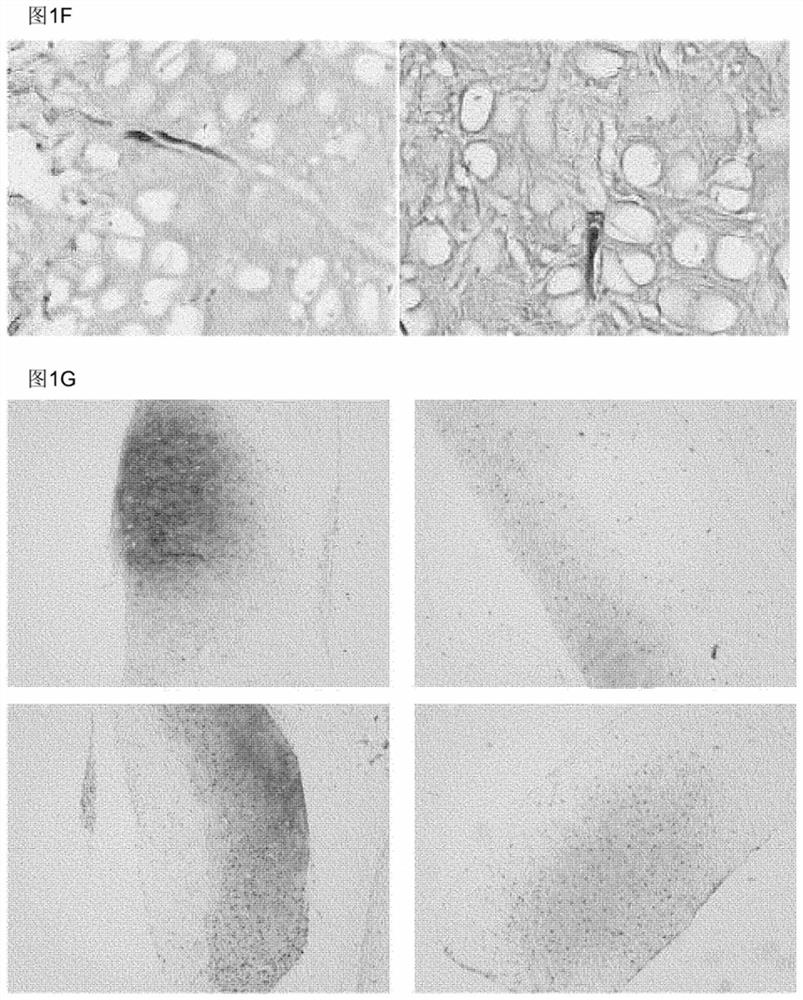
Elastin reduction allowing recellularization of cartilage implants
An elastin, recellularization technology, applied in the field of improving cartilage grafts, tissue implants, can solve the problem of not showing cell invasion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1: Recellularization with BMSCs by dynamic seeding
[0084] Wash the outer ear of a 12-week-old calf with water, and remove the perichondrium, skin, and muscle with a scalpel until only cartilage remains. Subsequently, biopsy samples with a diameter of 6 mm and a height of approximately 1 mm are obtained from the ear cartilage using a biopsy punch, so each sample has a volume of approximately 30 mm 3 .
[0085] Cells and elastin fibers were then removed using the following protocol, volumes given are for batches of approximately 20 samples unless otherwise stated. First, the samples were briefly rinsed three times with 40 ml. In some embodiments of the invention, 3696 Grade I deionized water (hereafter referred to as deionized water) is used to remove debris, such as loose pieces of skin, from the isolate. If necessary, samples were stored frozen in 20 ml of deionized water at -20°C. The samples were then sterilized with 20 ml of 5% hydrogen peroxide for 60 mi...
Embodiment 2
[0089] Example 2: In situ cartilage formation after injection
[0090] Certain areas of cartilage have difficulty reaching cells by migration, for example, the cartilage of the outer ear is surrounded by a dense layer of perichondrium. As shown by Utomo et al. 2015, whole ears can be decellularized using elastase. The perichondrium is tightly attached to the cartilage, and it is not feasible to remove it without damaging the cartilage structure.
[0091] To test whether recellularization of the whole ear is feasible, bovine ear cartilage samples were recellularized with chondrogenic cells by perichondrial injection without having to remove the perichondrium.
[0092] The external ear of a 12-week-old calf was washed with water, and the skin and muscle were removed with a scalpel until only the cartilage of the intact perichondrium remained. Subsequently, square biopsy samples with a side length of 15 mm are obtained from the ear cartilage using a scalpel, and each sample has...
Embodiment 3
[0097] Example 3: In vivo cartilage formation of joint models
[0098] To determine whether elastase-treated elastic cartilage is suitable for repairing cartilage defects, it was used to fill defects in an articular cartilage injury model. First, the outer ear of a 12-week-old calf was washed with water, and the perichondrium, skin, and muscle were removed with a scalpel until only cartilage remained.
[0099] Subsequently, a 6 mm diameter biopsy was obtained from the ear cartilage using a biopsy punch and cut to a height of approximately 0.4 or 1.2 mm.
[0100] The protocol used to remove cells and elastin fibers was the same as that described in Example 1. Volumes given are for batches of approximately 20 samples unless otherwise stated.
[0101] After removing cells and elastin fibers, place half of the sample in a 0.3 cm 2The bottom of the well (one sample / well, 96-well plate), and cover 500,000 chondrocytes in each sample, as a solution, add to 200 μl of chondrocyte ex...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com