Pharmaceutical composition for preventing and treating diabetic retinopathy
A technology for diabetic retina and composition, which is applied in the field of pharmaceutical compositions for proliferative diabetic retinopathy, can solve the problems that experimental research has not been widely carried out and the understanding is limited.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
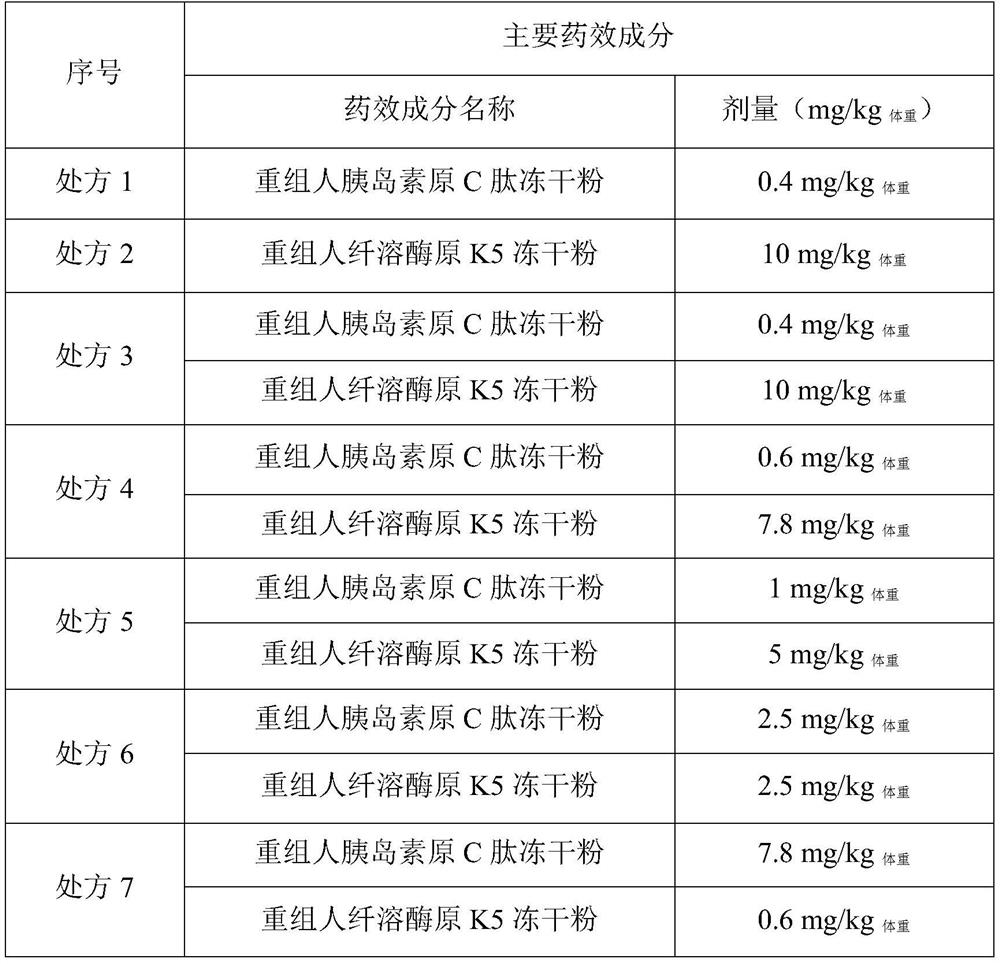
Embodiment 1
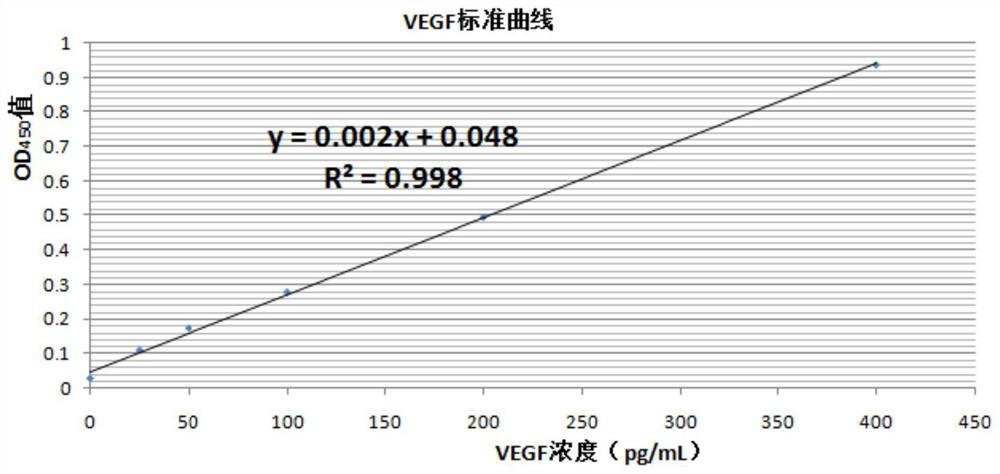
[0095] Example 1 VEGF ELISA of Rat Retinal Tissue and Measurement of Nitric Oxide NO Content in Eyeball Tissue
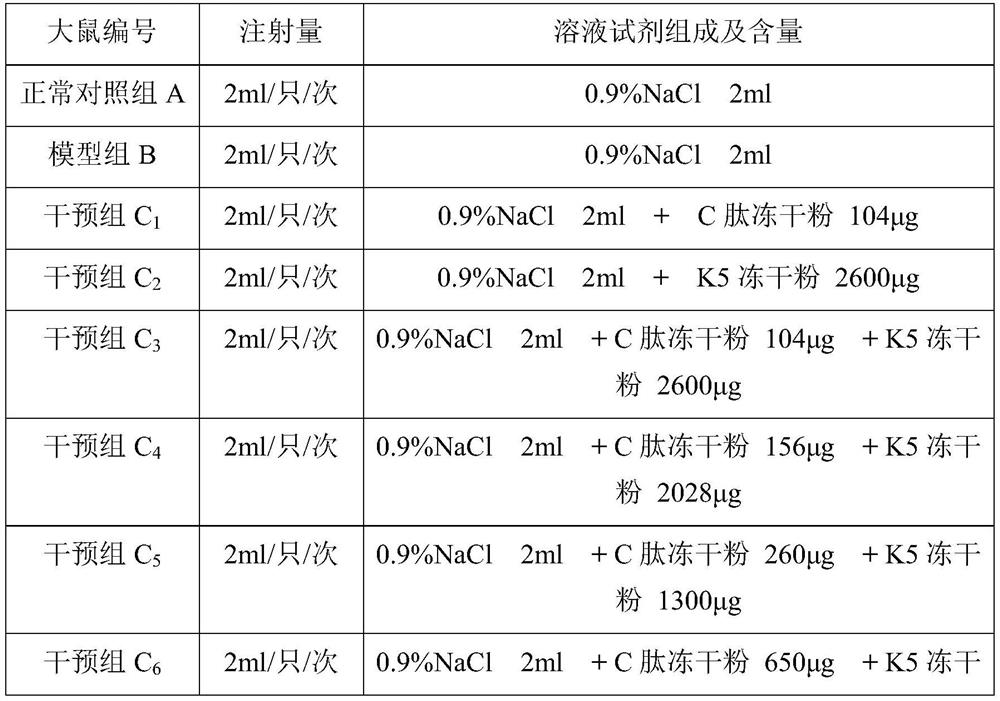
[0096] Materials: At the end of the twelfth week, 10 rats in each group were killed by dislocation of the neck, and the left and right eyeball tissues were removed. At the same time, blood samples were taken from the orbital vein after the left and right eyeball tissues were removed, and the eyeballs and blood samples were refrigerated according to the corresponding group number. The left and right eyeball tissues of each group of rats were dissected, and the retinal tissues were added to the pre-cooled homogenate medium, and the cells were broken to obtain tissue homogenate for detection of the expression level of VEGF in the retinal tissues. Blood samples were centrifuged to obtain serum for the determination of NO content.
[0097] Rat vascular endothelial growth factor (VEGF) ELISA kit was purchased from Shanghai Renjie Biotechnology Co., Ltd. Sensitivity: the d...
Embodiment 2
[0130] Example 2 Determination of Nitric Oxide NO Content in Eyeball Tissue
[0131] Determination method: microplate method
[0132] Kit: nitric oxide (NO) assay kit (Nanjing Jiancheng Institute of Bioengineering; specification: 96T; detection range: 0-600μmol / L; bottom detection line: 1.8μmol / L);
[0133] Instruments: 1. Spectrophotometer 2. Constant temperature water bath (incubation temperature is 37°C) 3. Vortex mixer 4. Desktop centrifuge 5. Micropipette
[0134] Operation process: operate according to the instruction manual.
[0135] Statistical analysis: The results of each group that conformed to the normal distribution were expressed as mean ± standard deviation. The comparison between multiple groups was performed by one-way analysis of variance, and the pairwise comparison between the means of each group was performed by the SNK-q test. The results are shown in the table.
[0136]The difference among the 9 groups was statistically significant (F=19.87, P0.05); t...
Embodiment 3
[0147] Example 3 Evans blue (evans blue) method to measure the permeability of the blood-retinal barrier
[0148] Material collection: The remaining 10 rats in each group were taken to detect the EB value.
[0149] Reagent: Evans Blue (Company: Beijing Jinming Biotechnology Co., Ltd.; serial number: E80004; Dye content: ≥85%)
[0150] Operation: Operate according to the instructions of the reagents.
[0151] Statistical analysis: The results of each group that conformed to the normal distribution were expressed as mean ± standard deviation. The comparison between multiple groups was performed by one-way analysis of variance, and the pairwise comparison between the means of each group was performed by the SNK-q test. The results are shown in the table.
[0152] The difference among the 9 groups was statistically significant (F=194.84, P0.05); There were statistically significant differences among the remaining groups (P<0.05).
[0153] The average content of Evans blue EB l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com