Application of glutamine in preparation of drug for inhibiting escherichia coli from generating gene mutation
A technology of Escherichia coli and glutamine, applied in the field of medicine, to reduce the probability of mutation, reduce the survival rate, and slow down the effect of drug resistance gene mutation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The in vitro passage experiment of embodiment 1 clinical sensitive bacterium and clinical drug-resistant bacterium
[0036] 1.1 Sample preparation
[0037] Pick clinically isolated Escherichia coli sensitive bacteria K12, S13, S14 and drug-resistant bacteria Y9, Y17, Y23 single clones (initial strain WT), inoculate them in LB liquid medium, culture at 37°C, 200rpm for 16h, and centrifuge at 8000g for 5min , collect the bacteria, and then suspend and wash the bacteria with sterile physiological saline, repeating three times. Resuspend the prepared bacteria with saline to OD 600 0.2, aliquot 5mL in a test tube for later use.
[0038] 1.2 Passage of clinically sensitive bacteria and clinical drug-resistant bacteria
[0039] Divide each strain into 2 groups, one group is ampicillin single-use group (AMP, abbreviated as A), the concentration of added antibiotics is K12: 0.003125mg / mL, S13: 0.003125mg / mL, S14: 0.0125mg / mL, Y9: 0.8mg / mL, Y17: 0.8mg / mL, Y23: 0.8mg / mL; the o...
Embodiment 2
[0040] Example 2 Sequence Analysis of Main Genes of Glutamine Metabolism Pathway and Purine Metabolism Pathway of Subcultured Bacteria
[0041]Glutamine metabolism ( carA, carB, gdhA, glnA), purine metabolism (purF, purB, purD, purE, purK, purH, purM, purN, purT, yfbR, rihB), dual regulatory system (cpxA, cpxR) and outer membrane antibiotic entry channel proteins (ompF) pathway main drug resistance gene sequence determination. The sequencing primers used are listed in Table 2.
[0042] Table 2 Sequencing Primers
[0043]
[0044]
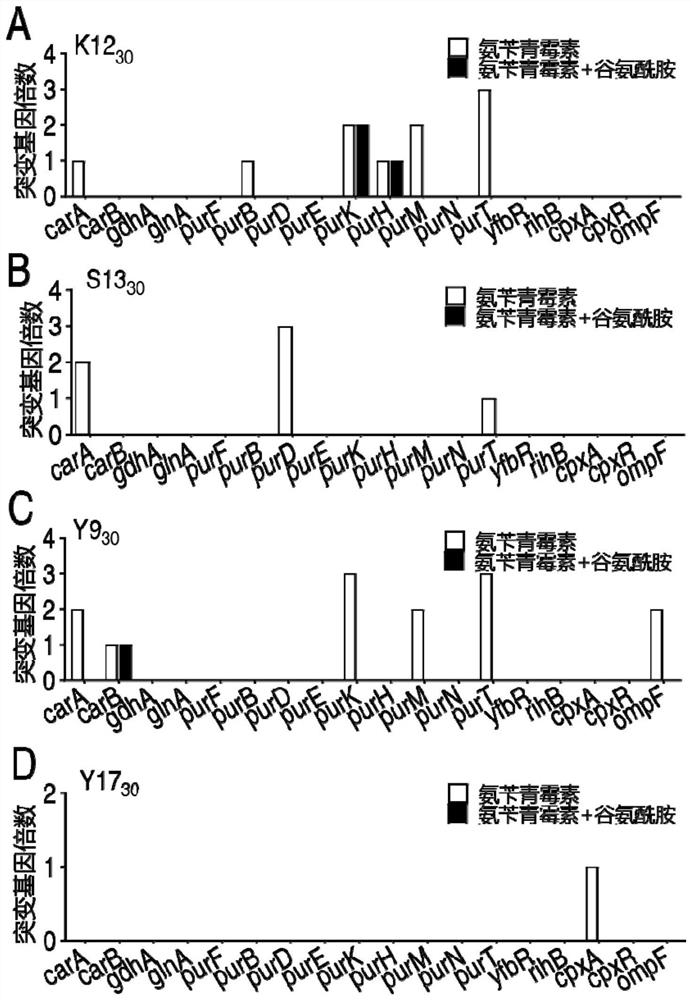
[0045] The sequence obtained after sequencing was analyzed by BLAST, and compared with the gene sequence of the corresponding bacterial initial strain, it was found that: for the K12 strain, 1 to 3 missense mutations were found in the genes such as carA, purB, purK, purH, purM, and purT in K12-30A point, while K12-30AG only found missense mutations in purK and purH ( figure 1 A) For Y9 strain, 1 to 3 missense mutation sites were found in c...
Embodiment 3
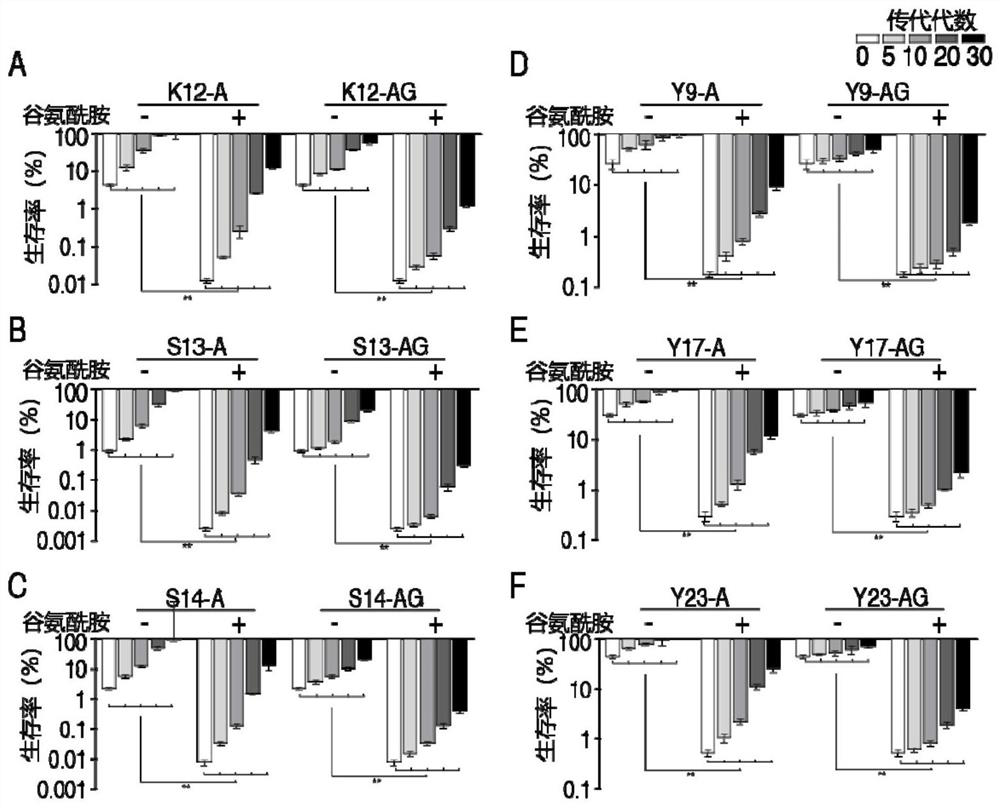
[0046] Example 3 In Vitro Passage Strain Drug Resistance Research
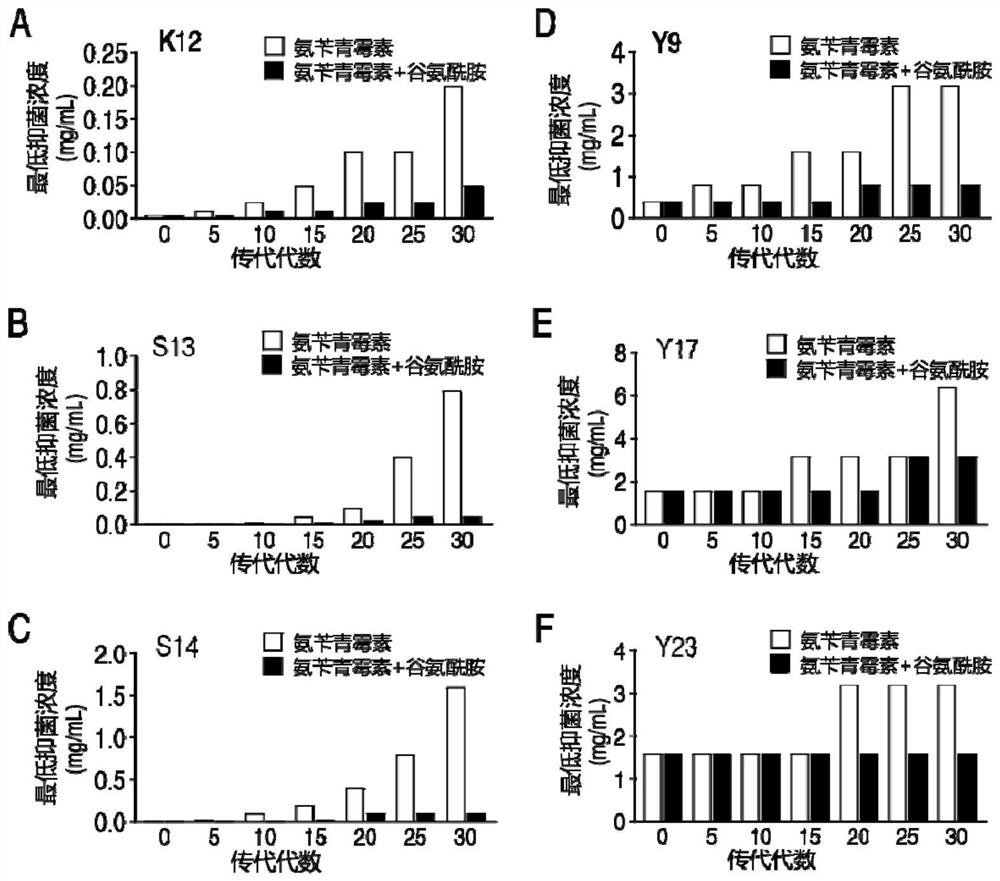
[0047] 3.1. Determination of minimum inhibitory concentration
[0048] Get 6 kinds of bacteria (K12, S13, S14, Y9, Y17, Y23) according to the 2 kinds of passage ways of embodiment 1 (antibiotics are used alone or antibiotics and glutamine are used in combination) to obtain the 0th, 5th, 10th, 15th, 20, 25, and 30 subcultured bacteria were used to measure the minimum inhibitory concentration of ampicillin by the micro-broth dilution method, and the specific methods were as follows:
[0049] The subcultured bacteria of each generation were inoculated in LB liquid medium, and cultured at 37°C and 200rpm for 16h; respectively transferred to 5mL LB medium and cultured to OD 600 0.5, diluted 10 times; ampicillin was added to the 1st to 11th columns in a sterile 96-well plate, 100 μL per well, and no antibiotics were added to the 12th column as a control; 10 μL of bacterial solution (approx. 10 5 CFU bacteria, tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com