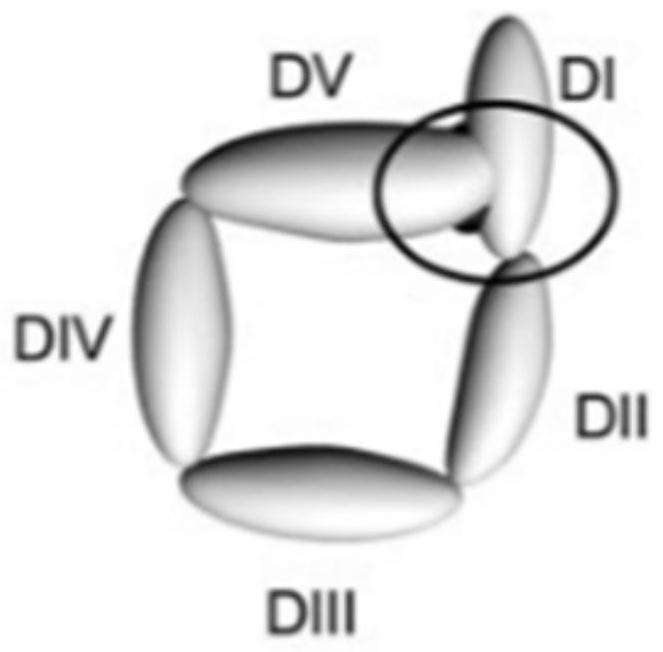
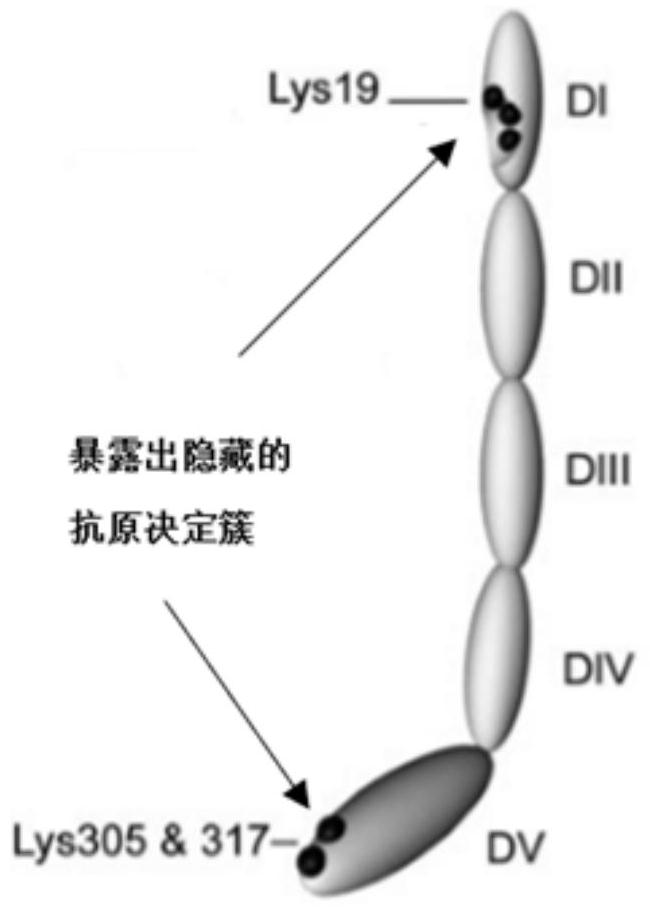
Method for opening the V domain of natural β2 glycoprotein I and antigen prepared by using the method, application and kit
A glycoprotein and structural domain technology, applied in the field of antigen preparation, can solve the problems of low sensitivity, high cost, and poor stability, and achieve the effect of simple and efficient method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] A method for natural β2-glycoprotein I, V domain is opened, comprising the steps of:
[0067] Step 1. Select a molecular weight of 50kDa human type natural β2-glycoprotein I.
[0068] Step 2. The native β2 glycoprotein I containing 1.15M NaCl, pH is in 20mM HEPES 11.5 (hydroxyethylpiperazine B sulfuric acid) buffer, dialyzed at 4 ℃ 48 hours, the medium was changed at 24 hours.
[0069] Obtained in Step 3. Step 2 β2 glycoprotein I containing 0.15M NaCl, pH 7.4 to 20mM HEPES buffer, and dialyzed at 4 ℃ 8 hours.
[0070] Obtained in step 4. Step β2 glycoprotein I 3 was added bacterial lipid A, disaccharide I obtained protein mass β2 and bacterial lipid A ratio of 500: 1, vortexed vessel was placed at 37 ℃, the reaction for 1 hour at 1000rpm.
[0071] Obtained in step 5. Step 4 I do β2 glycoprotein isolated and purified by HiTrap Chelating column.
[0072] Step 6. Step 5 obtained after purification β2 glycoprotein I quantified by UV spectrophotometer, containing 0.15M NaCl, pH 7....
Embodiment 2
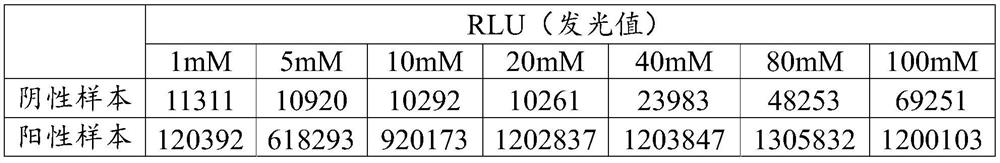
[0103] The present embodiment provides a method for opening a natural β2-glycoprotein I, V domains, different from Example 1 in that, in step 2, HEPES buffer concentration of 10mM.
Embodiment 3
[0105] The present embodiment provides a method for opening a natural β2-glycoprotein I, V domains, different from Example 1 in that, in step 2, HEPES buffer concentration of 40mM.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com