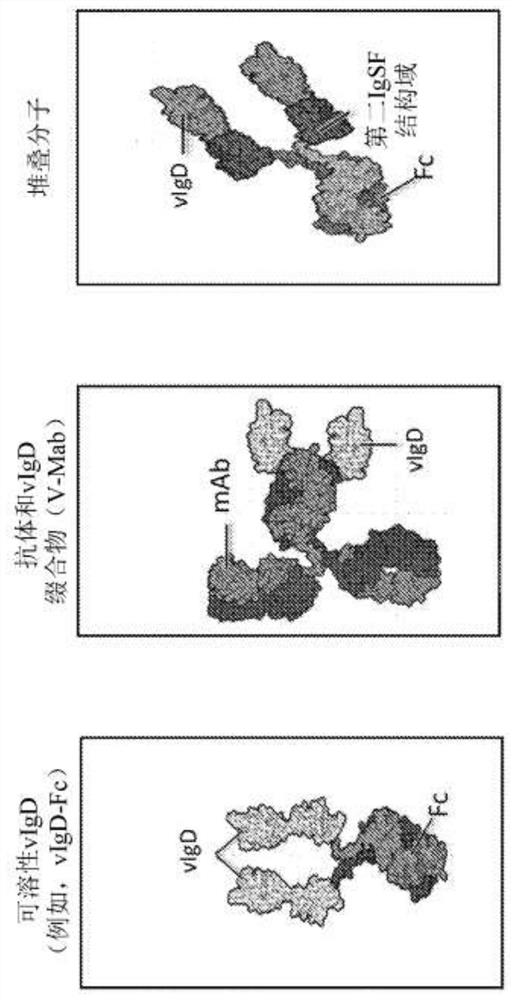
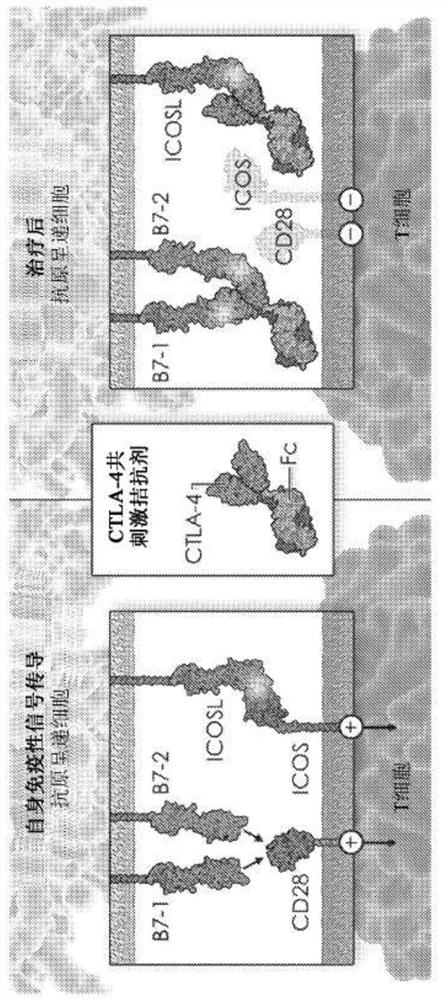
Ctla-4 variant immunomodulatory proteins and uses thereof
A technology of CTLA-4, variants, applied in the field of preparation and use of compositions of such proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0679] Generation of mutant DNA constructs of the IgSF domain
[0680] A mutant DNA construct of the human CTLA-4 IgSF domain was generated for translation and expression on the surface of yeast as a yeast display library.
[0681]A library containing random amino acid substitutions was constructed to identify variants of the ECD of CTLA-4 based on the wild-type human CTLA-4 sequence shown in SEQ ID NO: 2 below: .
[0682] KAMHVAQPAVVLASSRGIASFVCEYASPGKATEVRVTVLRQADSQVTEVCAATYMMGNELTFLDDSICTGTSSGNQVNLTIQGLRAMDTGLYICKVELMYPPPYYLGINGTQIYVIDPEPPCPDSD (SEQ ID NO: 2)
[0683] DNA encoding wild-type CTLA-4 ECD was cloned between the BamHI and KpnI sites of the modified yeast display vector pBYDS03 (Life Technologies). By error-prone PCR, using supplemented with MnCl 2 Genemorph II Kit (Agilent, USA) and ECD-specific oligonucleotides that overlapped the pBYDS03 cloning vector by 40 bp outside and including the BamHI cloning site and the KpnI cloning site to introduce mutations. Th...
example 2
[0686] Introduction of DNA library into yeast
[0687] The CTLA-4 DNA library generated in Example 1 was introduced into yeast using electroporation. Briefly, electroporation-competent cells of yeast strain BJ5464 (ATCC.org; ATCC No. 208288) were prepared and tested on a Gene Pulser II (Biorad) Electroporation was performed on DNA prepared for electroporation as described above (Colby, D.W. et al., 2004 "Methods Enzymology" 388, 348-358). The only exception is that transformed cells were grown in non-inducible minimally selective SCD-Leu medium to accommodate the LEU2 selectable marker carried by the modified plasmid pBYDS03. One liter of SCD-Leu medium consists of 14.7 g sodium citrate, 4.29 g citric acid monohydrate, 20 g dextrose, 6.7 g yeast nitrogen base and 1.6 g yeast synthesis deficient medium supplement without leucine (yeast synthetic drop-out media supplement). Before use, filter-sterilize the medium using a 0.22 μm vacuum filter unit.
[0688] The library size ...
example 3
[0690] yeast selection
[0691] Yeast expressing affinity-modified variants of CTLA-4 are selected for ICOSL and / or CD86.
[0692] A number of cells equal to at least 10 times the estimated library size were thawed from individual library stocks, suspended to 1.0 x 10E6 cells / ml in non-inducing SCD-Leu medium, and grown overnight. The next day, an amount of cells equal to 10 times the library size was centrifuged at 2000 RPM for 2 minutes and resuspended to 5.0 x 10E6 cells / ml in inducible SCDG-Leu medium. One liter of SCDG-Leu induction medium consists of 5.4 g of Na dissolved in water 2 HPO 4 , 8.56 g NaH 2 PO 4 ·H 2 0. 20 g galactose, 2.0 g dextrose, 6.7 g yeast nitrogen base and 1.6 g yeast synthesis deficient medium supplement without leucine and sterilized through a 0.22 μm membrane filter unit. Cultures were grown in induction medium at room temperature for 1 day to induce expression of library proteins on the surface of yeast cells.
[0693] The induced yeast li...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com