Preparation method of tert-butyl 3-oxocyclobutyl carbamate
A technology of oxocyclobutylcarbamate and tert-butyl ester, applied in the field of medicine, can solve the problems of being unsuitable for industrial production, low yield and the like, and achieve the effects of low cost, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
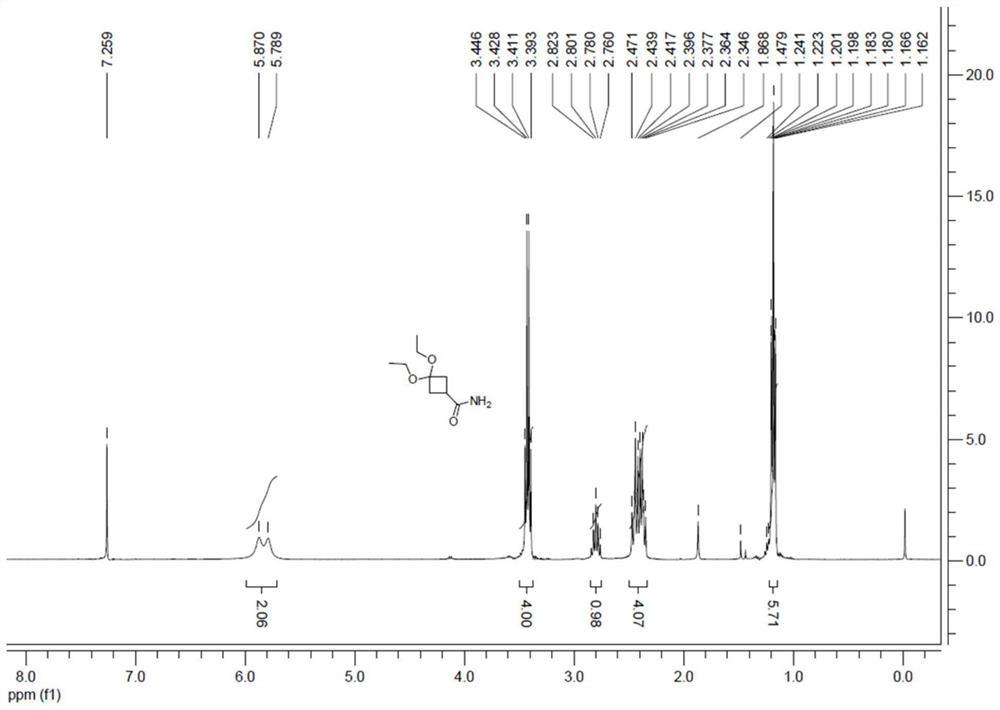
[0032] The preparation of embodiment 1 compound III
[0033] Add 50 g of compound IV to 200 mL of ethanol, then add 3.8 g of p-toluenesulfonic acid, cool to 0-5°C, add 79 g of triethyl orthoformate dropwise, and react at room temperature for 20 h after the drop is completed, and follow TLC until the reaction is complete. Extract and concentrate to dryness with dichloromethane, add 50mL methyl tert-butyl ether to make a slurry, filter, wash, and dry to obtain 75g of white solid with a yield of 91% and a purity of 98%. 1 For H NMR data see figure 1 .
Embodiment 2
[0034] The preparation of embodiment 2 compound III
[0035] Add 50 g of compound IV to 200 mL of ethanol, then add 2.2 g of concentrated sulfuric acid, cool to 0-5°C, add 79 g of triethyl orthoformate dropwise, and react at room temperature for 20 h after the drop is completed, followed by TLC until the reaction is complete. Extract and concentrate to dryness with dichloromethane, add 50mL methyl tert-butyl ether to make a slurry, filter, wash, and dry to obtain 71g of white solid with a yield of 86% and a purity of 98%.
Embodiment 3
[0036] The preparation of embodiment 3 compound III
[0037] Add 50g of compound IV to 200mL of methanol, then add 3.8g of p-toluenesulfonic acid, cool to 0-5°C, slowly add 57g of trimethyl orthoformate dropwise, and react at room temperature for 20h after the drop is completed, and follow TLC until the reaction is complete. Extract and concentrate to dryness with dichloromethane, add 50 mL of methyl tert-butyl ether to make a slurry, filter, wash, and dry to obtain 62 g of white solid with a yield of 88% and a purity of 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com