Pharmaceutical composition comprising timolol
A composition, a technology of a drug, applied in the field of topical application in the eye, raising intraocular pressure, treating glaucoma, high intraocular pressure and/or symptoms related thereto, capable of solving not representing the best choice, poor solubility, infeasible And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0187] The following examples serve to illustrate the invention; however, these should not be construed as limiting the scope of the invention. Example 1: Preparation of Timolol Suspension
[0188] 109,34 mg of timolol maleate CAS number 26921-17-5 (LKT Labs; 99.5% purity) was introduced into a 25 mL vessel fitted with a 2 mm diameter stainless steel ball. Then, 8 mL of 1-perfluorohexyl-octane (F6H8) was added, the vessel was closed and ground for 3 hours at 150 rpm with a planetary ball mill (PM 100, Retsch GmbH, Germany) at 10 minute intervals (variation method). After trituration, the suspension thus formed was transferred to a glass vial, shaken on a Vortex shaker for a minimum of 30 seconds, and sealed. Considering that 1.3668 mg timolol maleate corresponds to 1.0 mg timolol free base, a 1.0% (w / v) suspension of timolol in F6H8 (10 mg / mL) was obtained.
[0189] Following the same procedure as above, a 15 mg / ml timolol suspension was prepared.
[0190] Dilute the suspen...
example 2
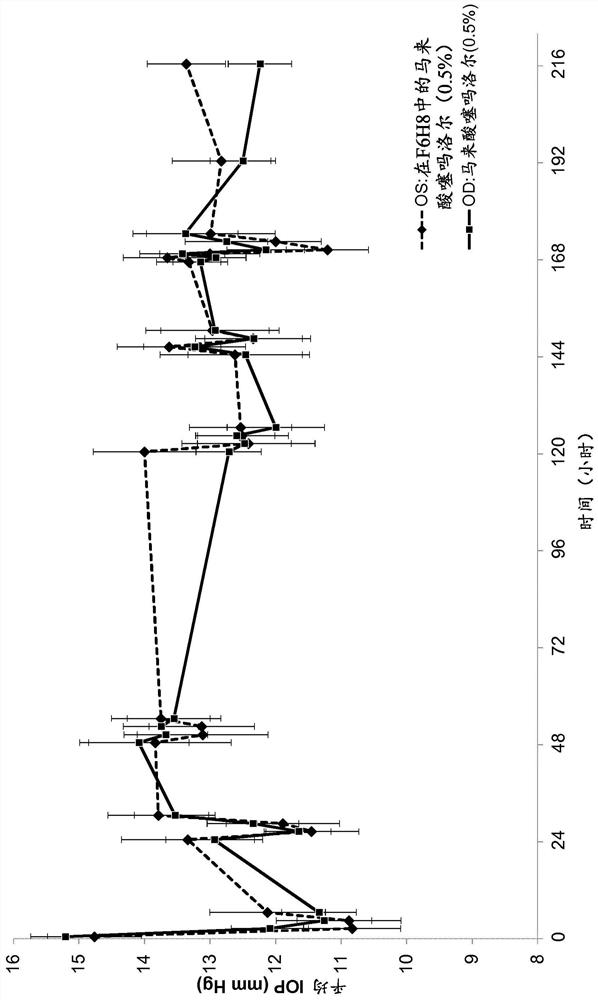
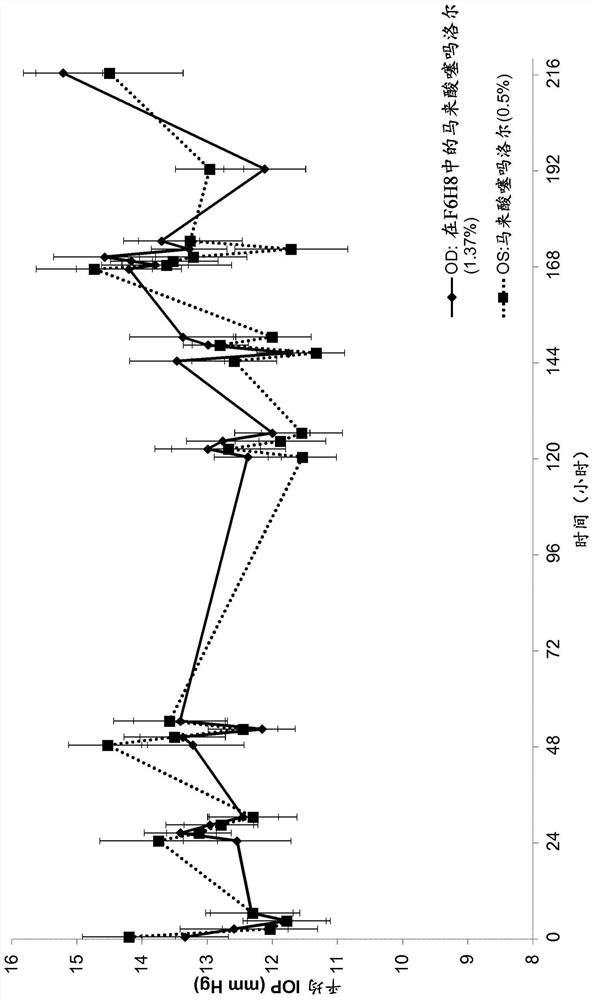
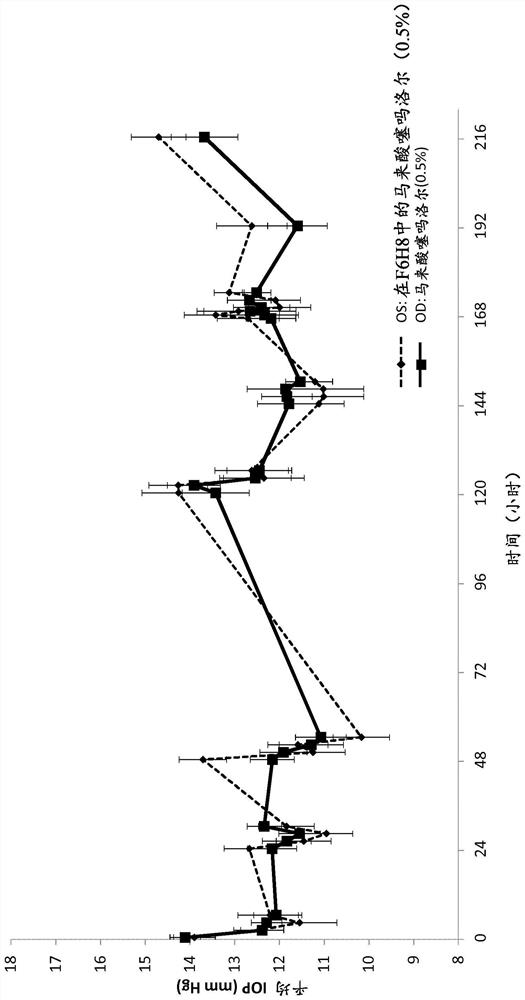
[0201] Example 2: Measurement of Intraocular Pressure (IOP) in Animal Studies
[0202] To evaluate the pharmacodynamics of the ability of a composition comprising timolol and a semifluorinated alkane to reduce IOP (intraocular pressure) compared to a commercially available solution of timolol administered in aqueous solution, a blood Animal studies of normal dogs. The study setup and design are as follows.
[0203] Dogs were selected for participation in the study based on general health, body weight, ophthalmic examination results, response to IOP challenge, and the following criteria:
[0204] - healthy, normal ocular surface;
[0205] - No invasive eye surgery for at least one month prior to the study; especially surgery generally involving the cornea or anterior segment;
[0206] - No topical or systemic corticosteroid therapy for at least one month;
[0207] - Clearance of drug from previous topical ocular studies comparable to the typical washout period (at least one...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com