N-formamido pyrazoline derivative serving as P2X3 receptor antagonist and application thereof
A technology of carboxamidopyrazolines and derivatives, which is applied in the field of medicine and can solve the problems of insufficient patient compliance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] One, the preparation method of main intermediate
[0060] 1. Preparation of intermediate N-formyl chloride pyrazoline derivative (A)
[0061] 1). Synthesis of 4-(N-methylacetamido)-3-(4-methylphenyl)-4,5-dihydro-1H-pyrazole-1-carbonyl chloride (A-1)
[0062]
[0063] Step 1. Synthesis of N-methyl-N-(2-oxo-2-(4-methylphenyl)ethyl)acetamide (A-1a)
[0064] 30% Methanol solution (7.29 g, 70.41 mmol) was added into acetonitrile (20 mL), N 2 Under protection, lower the temperature to -15°C to -10°C, slowly add dropwise a solution of 4-methyl-α-bromoacetophenone (5g, 23.47mmol) in acetonitrile (40mL), and keep at -15°C after the addition is complete Stir at ~-10°C for 20 min. The temperature of the reaction solution was raised to -5°C to 0°C, ice water (60mL) was added dropwise, stirred for 5min, ethyl acetate (30mL) was added, stirred for 5min, the layers were allowed to stand, and the aqueous phase was extracted with ethyl acetate (20mL×2 ), combined the organic phas...
preparation Embodiment 1
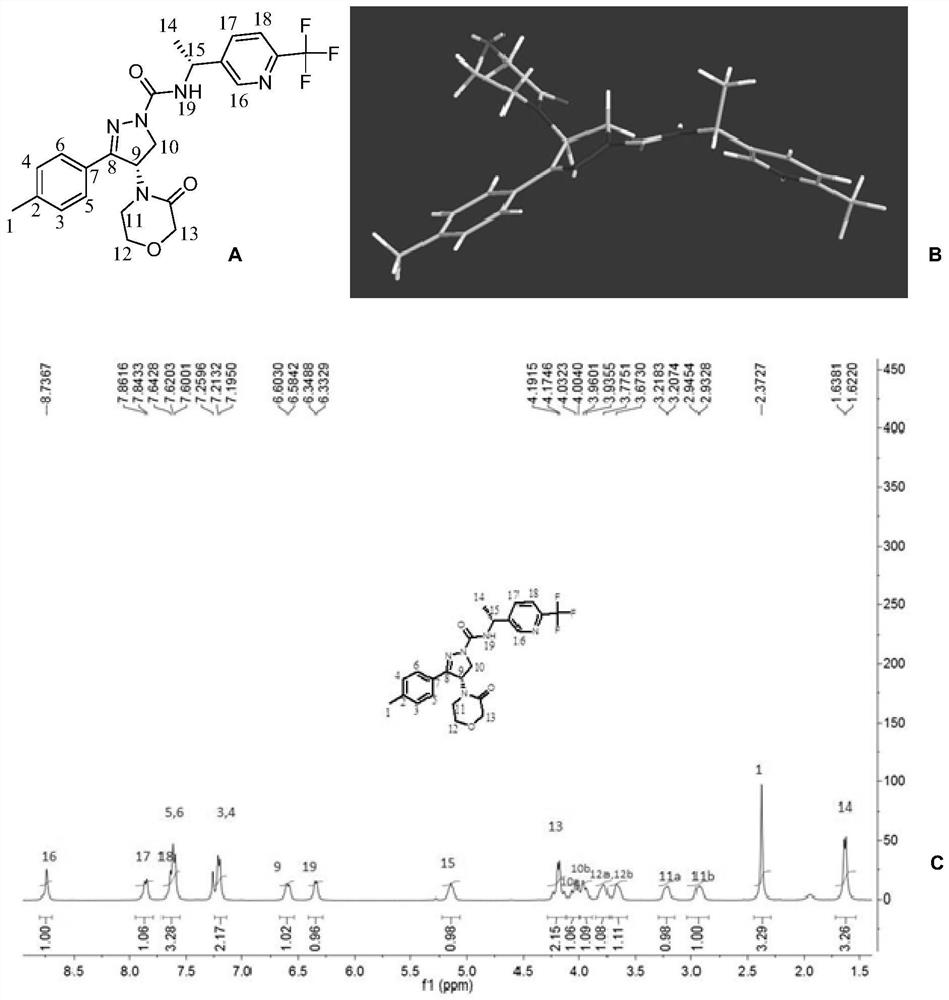
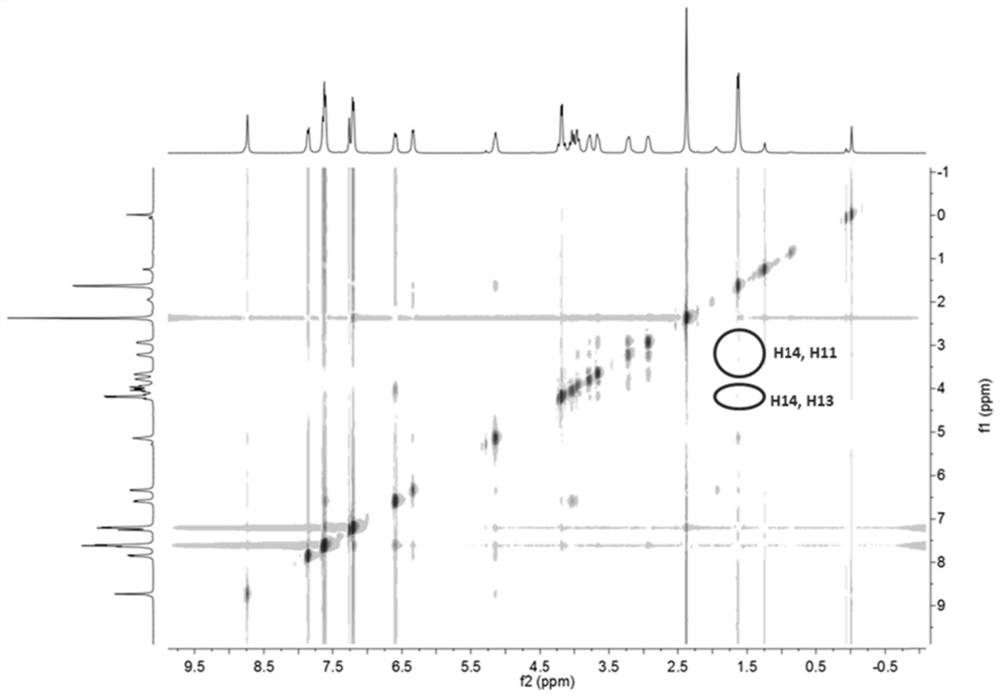
[0130] Preparation Example 1. (S)-4-(3-oxomorpholin-4-yl)-3-(4-methylphenyl)-N-((R)-1-(6-(trifluoro Synthesis of methyl)pyridin-3-yl)ethyl)-4,5-dihydro-1H-pyrazole-1-carboxamide (Compound 1)
[0131]
[0132] Synthetic steps: compound A-2 (1.05g, 3.26mmol), compound B-9 (0.62g, 3.26mmol) are added in methylene chloride (10mL), then add N,N-diisopropylethylamine ( DIPEA, 1.26g, 9.75mmol), stirred overnight at room temperature, added 0.5N hydrochloric acid (10mL) and stirred for 10min. Stand to separate the layers, separate the organic phase, extract the aqueous phase with dichloromethane (10mL×3), combine the organic phases, and wash with anhydrous Na 2 SO 4 Drying, filtration, the filtrate was concentrated to dryness under reduced pressure, and the residue was separated by silica gel column chromatography (PE:EA=3:1~1:3, v / v) to obtain the title compound 1 with a yield of 32% and its R , R-epimer 1a, yield 29%.
[0133] The structures of the title compound 1 and its epi...
preparation Embodiment 2
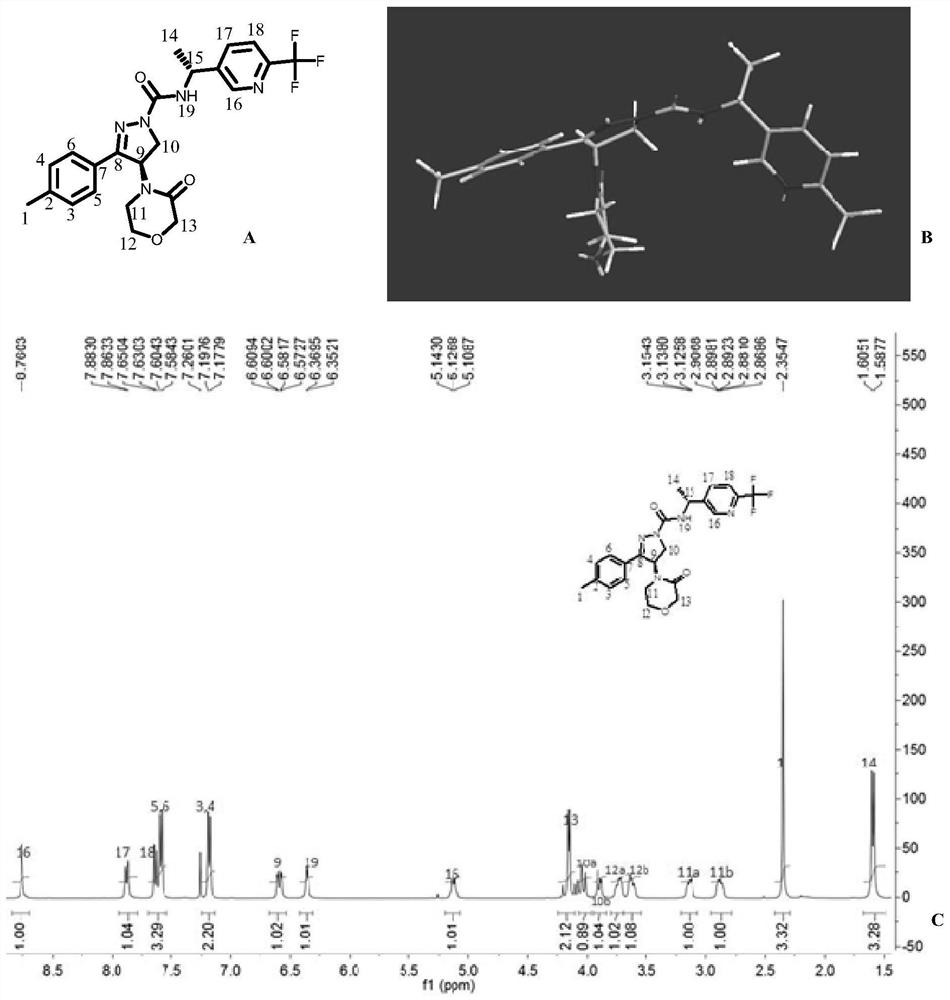
[0139] Preparation Example 2. (S)-4-(4-methyl-2-oxopiperazin-1-yl)-3-(4-methylphenyl)-N-((R)-1-( Synthesis of 4-chlorophenyl)ethyl)-4,5-dihydro-1H-pyrazole-1-carboxamide (compound 2)
[0140]
[0141] Synthesis steps: Compound B-2 (500mg, 2.63mmol) was added in dichloromethane (5mL), cooled to 0°C, N 2 Add triphosgene (370mg, 1.25mmol) under protection, Et 3 N (660mg, 6.55mmol), raised to room temperature and stirred for 1h. The reaction solution was poured into water, extracted with dichloromethane (10mL×2), and the organic phase was washed with anhydrous Na 2 SO 4 Dry, filter, and recover the solvent under reduced pressure to obtain crude isocyanate, which is directly used in the next reaction without further purification.
[0142]Compound 1-(3-(4-methylphenyl)-4,5-dihydro-1H-pyrazol-4-yl)-4-methylpiperazin-2-one (500mg, 1.80mmol), Crude isocyanate (410mg, 2.27mmol), K 2 CO 3 Added to acetone (10mL), N 2 Protected, stirred at room temperature for 2h, TLC showed th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com