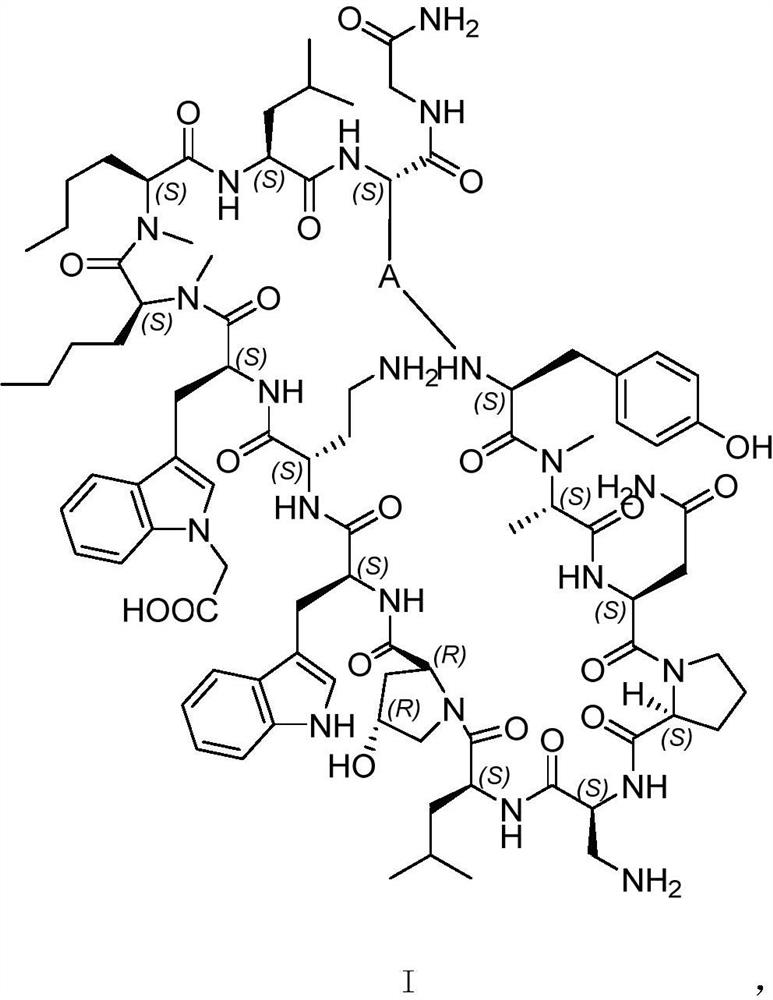
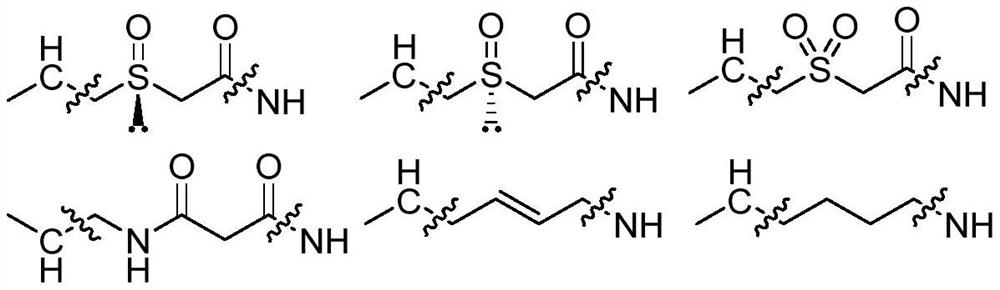
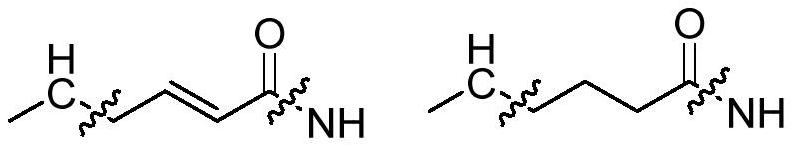PD-1/PD-L1 polypeptide inhibitor and medical application thereof
A technology of inhibitors and cyclic peptide compounds, applied in the field of medicinal chemistry, can solve the problem of slow progress of peptide inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
[0035]According to the aforementioned "resin swelling step", after the resin is swollen, according to the "condensation step", the selected amino acids are respectively Fmoc-L-Gly-OH, Fmoc-L-Cys(Trt)-OH, Fmoc-L-Leu-OH, Fmoc-L-[N-Me]Nle-OH, Boc-L-Trp-OH, Fmoc-L-Trp(Boc)-OH, Fmoc-L-Dab(Boc)-OH, Fmoc-L-[O- tBu]Hyp-OH, Fmoc-L-Dap(Boc)-OH, Fmoc-L-Pro-OH, Fmoc-L-Asn(Trt)-OH, Fmoc-L-[N-Me]Ala-OH, Fmoc -L-[O-tBu]Tyr-OH. After the condensation is completed, according to the aforementioned "peptide freeing step", select the corresponding amino acid to obtain the crude product of the linear peptide precursor. 60 mg of the crude product was dissolved in a total of 600 mL of acetonitrile:0.1M ammonium carbonate=1:1 (volume ratio), and the reaction was stirred at room temperature for 5 hours. The reaction solution was spin-dried and freeze-dried to obtain a powder mixture. The mixture was dissolved in acetonitrile: water = 1:1 (volume ratio), and the pure cyclic peptide precu...
Embodiment 2
[0037]
[0038] According to the method in the aforementioned "Example 1", t R = 27.251 min. HRESIMS:[M+H] + = 1916.9601.
Embodiment 3
[0040]
[0041] According to the synthesis of the aforementioned Example 1, the oxidation step was replaced by: taking 5 mg of the pure precursor and dissolving it in 500 μL of methanol:water=1:1 (volume ratio). 0.5 mg of potassium peroxymonosulfonate was added to the mixture, and stirred at room temperature. The reaction can be judged to be complete by RP-HPLC. Ascorbic acid was added to the reaction solution to destroy peroxide, and the product could be obtained according to the aforementioned "RP-HPLC separation step" after the reaction solution was freeze-dried. t R = 20.230 min. HRESIMS:[M+Na] + = 1896.9402.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com