Method for heteronuclear quantitative determination using nmr-spectroscopy, reference substances therefor, and method for determining the degree of deuteration of a deuterated compound
A technology for quantitative determination of compounds, applied in the direction of measurement using nuclear magnetic resonance spectrum, magnetic resonance measurement, and measurement using magnetic variables, which can solve the problems of large measurement uncertainty, inability to obtain, failure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0133] The determination of sodium in embodiment 1-heparin sodium
[0134] In the following, the determination of sodium in heparin sodium is provided as an example of the method of the present invention, heterokaryon quantitation.
[0135] For this purpose, the sodium content of 16 different sodium-heparin solution samples (S1-S16) was determined by the method according to the present invention (NMR) and compared with the method commonly used for this purpose by atomic absorption spectroscopy (AAS). The obtained values are compared.
[0136] To prepare samples, weigh a defined amount of Na-heparin and dissolve in a defined volume of D 2 O middle. Alternatively, a defined amount of the appropriate deuterated solvent can also be weighed. As deuterated solvent, DMSO-d 6 can be used as D 2 Alternatives to O.
[0137] In particular, the determination of sodium by NMR according to the present invention is required in the European and US Pharmacopoeias 1 Additional experime...
Embodiment 2
[0147] The mensuration of embodiment 2-NMR solvent deuteration degree
[0148] Suitable solvents for NMR experiments must contain very high levels of deuterium 2 H, instead of the naturally more abundant hydrogen atoms 1 H. For conventional NMR experiments, a specification of "very high" is sufficient, i.e. >99%D. The remaining 1% is 1 H.
[0149] Knowing this degree of deuteration is important for the heteronuclear quantitative NMR method according to the present invention. In the case of solvents with a very high degree of deuteration, e.g. >99.95% deuterated D 2 O, 0.05% 1 The ratio of H is unimportant and only contributes to the uncertainty of the assay. To accurately determine the degree of deuteration, NMR spectroscopy is a natural fit, as it can be used to measure 2 H(D) and 1 Hboth. The integral ratio D / H is directly proportional to the degree of deuteration. The necessary calibration is done by adding the same non-deuterated solvent and making the appropria...
Embodiment 3
[0159] Example 3 - Determination of deuterated NMR solvents with other deuterated solvents whose deuterated degree is known
[0160] In this example 3, deuterated CDCl was measured 3 degree of deuteration. For this, deuterated CDCl was prepared in a mixing ratio of about 1:1 3 and DMSO-d for Example 2 6 (Deuterated degree 99.57%) mixture. Since the mixing ratio does not need to be known exactly, it can be weight-related or volume-related. Other mixing ratios can also be used as long as meaningful NMR spectra can be obtained. Measure the resulting mixture of 2 H and 1 H NMR (one single pulse each).
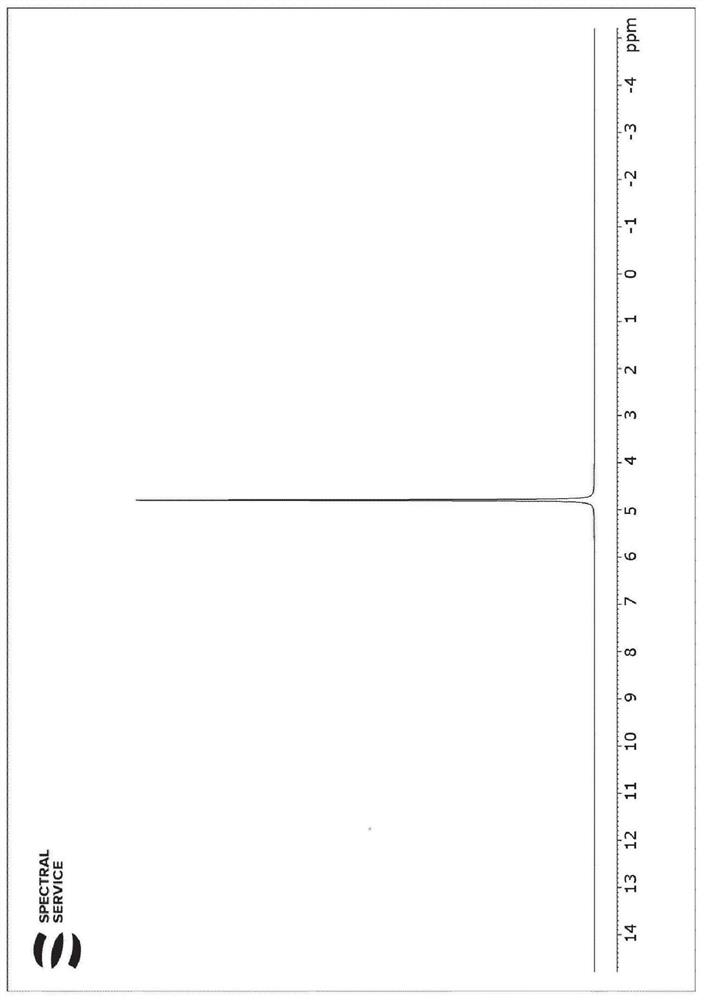
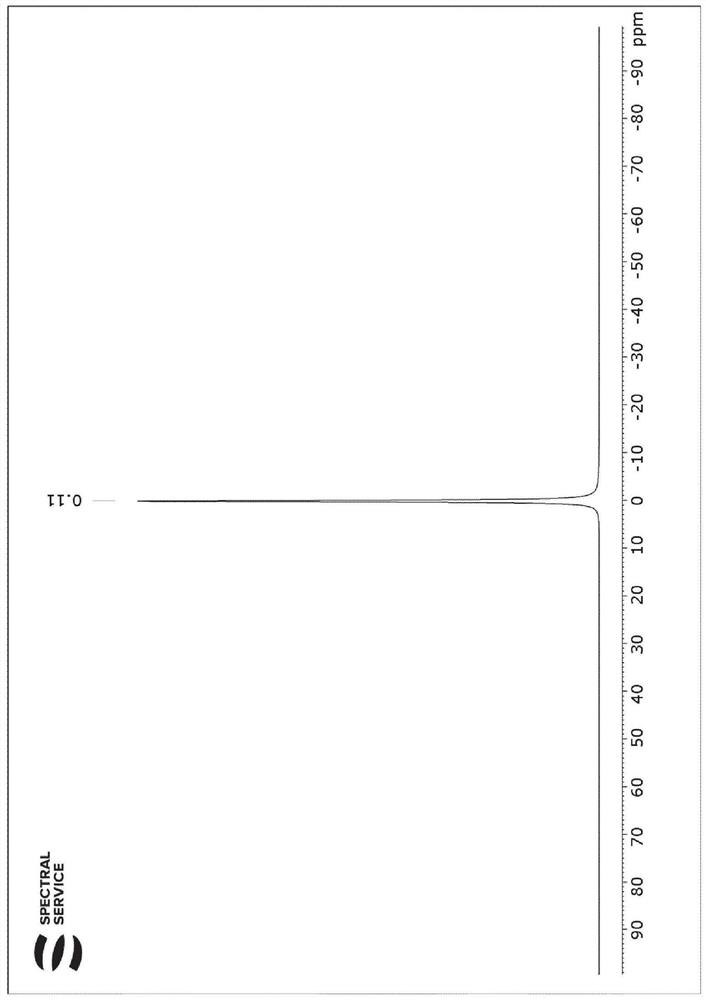
[0161] Figure 7 The prepared CDCl is shown on the left 3 and DMSO-d 6 mixture 1 H NMR spectra and the corresponding 2 H NMR Spectrum.
[0162] Will come from DMSO-d 6 The integral ratio D / H of the spectrum is the same as that from CDCl 3 Integral ratio D / H comparison of the spectra, taking into account the known degree of protonation (DMSO-d 6 {H} DMSO ), calcul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com