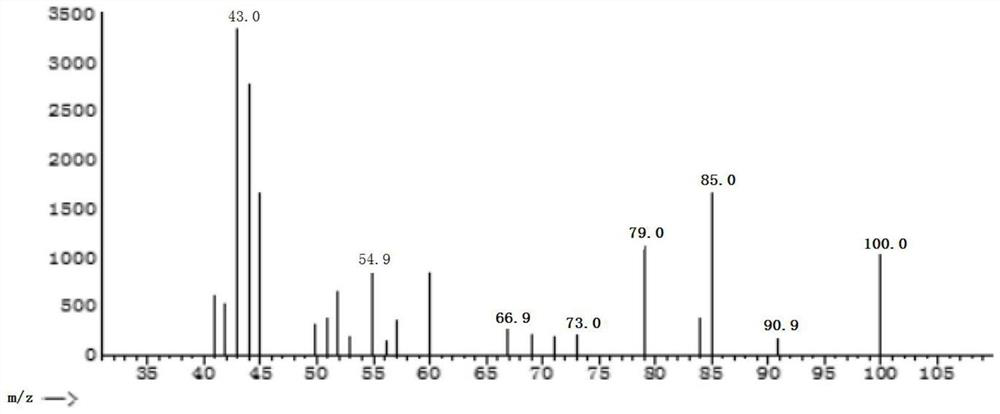
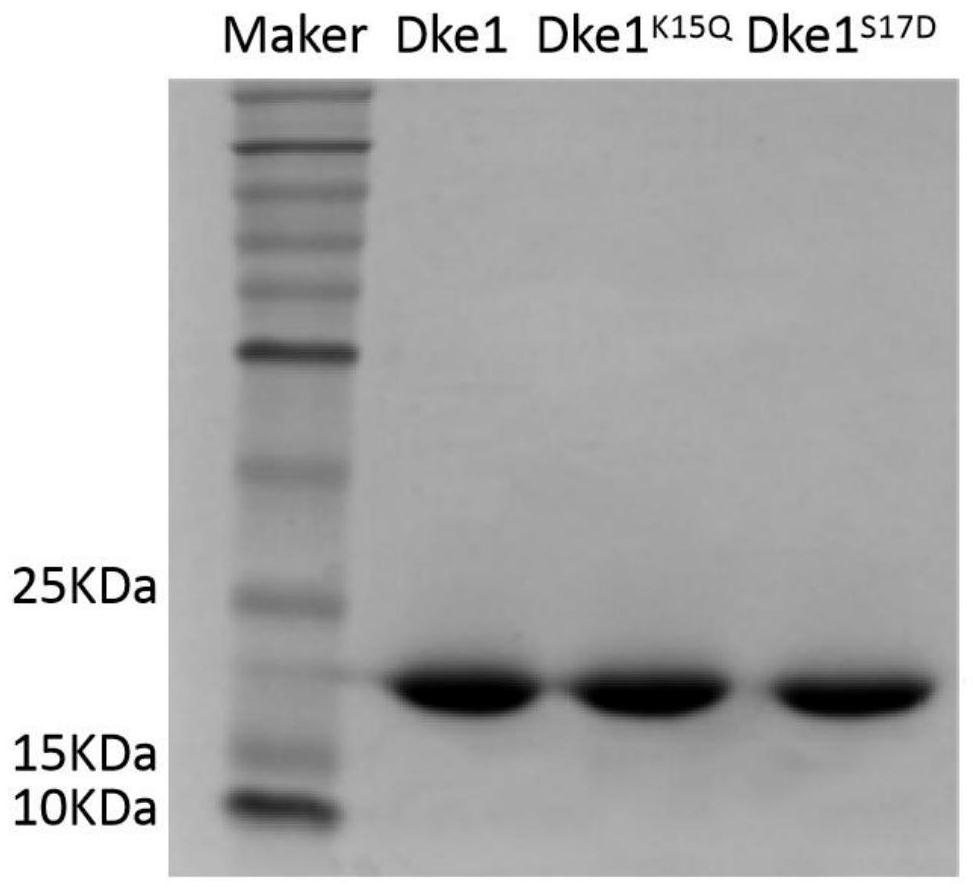
Method for generating acetylacetone through extracellular enzyme reaction
A technology of acetylacetone and extracellular enzymes, applied in the field of bioengineering, can solve the problems of low production of acetylacetone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0034] Preparation example Preparation of the acetylacetone lyase mutant coding gene sequence Dke1 that improves the synthesis efficiency of acetylacetone K15Q
[0035] Codon optimization was performed on the acetylacetone lyase gene sequence derived from Acinetobacter johnsonii, and the gene (SEQ ID NO.4) was synthesized by Suzhou Jinweizhi Company. Using the synthesized gene as a template, use site-directed mutagenesis to design primers.
[0036] The upstream fragment of the dke1 gene (primers: F: CGGGATCCGATGGACTACTGCAACA and R: TTGTTGTCAGAGATTTGAACGTATTCTT) and the downstream fragment (primers: F: AAGAATACGTTCAAATCTCTGACAAAA and R: CGGAATTCTTAAGCAGCTTCGTTTTTGGTA) were obtained by PCR amplification, and then the target fragment was recovered using a recovery kit. The obtained upstream and downstream fragments were used as substrates, and the acetylacetone lyase mutant gene sequence Dke1 in which the 15th lysine was changed to glutamine was obtained by bridging PCR (primers...
Embodiment 1
[0038] Dke1 will be obtained K15Q The fragment and plasmid pETDuet-1 were digested with BamHI and EcoRI, and the digested product was recovered; then ligated: the recovered vector and the dke1 gene fragment were ligated at a molar ratio of 1:5 at 16°C for more than 6 hours; the ligated product was recovered and improved The expression vector of the gene encoding the acetylacetone lyase mutant of acetylacetone synthesis efficiency, named pETDuet-Dke1 K15Q .
[0039] The obtained vector pETDuet-Dke1 K15Q Introduce E.coli BL21(DE3) competent cells, spread in the -1 Ampicillin LB solid plate; place the coated plate in a constant temperature incubator at 37°C, and continue to culture until a single colony grows. Pick a single clone and streak it on a solid LB plate, continue culturing in a 37°C constant temperature incubator until the single clone grows again, use the single clone as a template, and verify it by colony PCR (primers are F: GATGCGTCCGGCGTAGAGC and R: GCTAGTTATTGCT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com