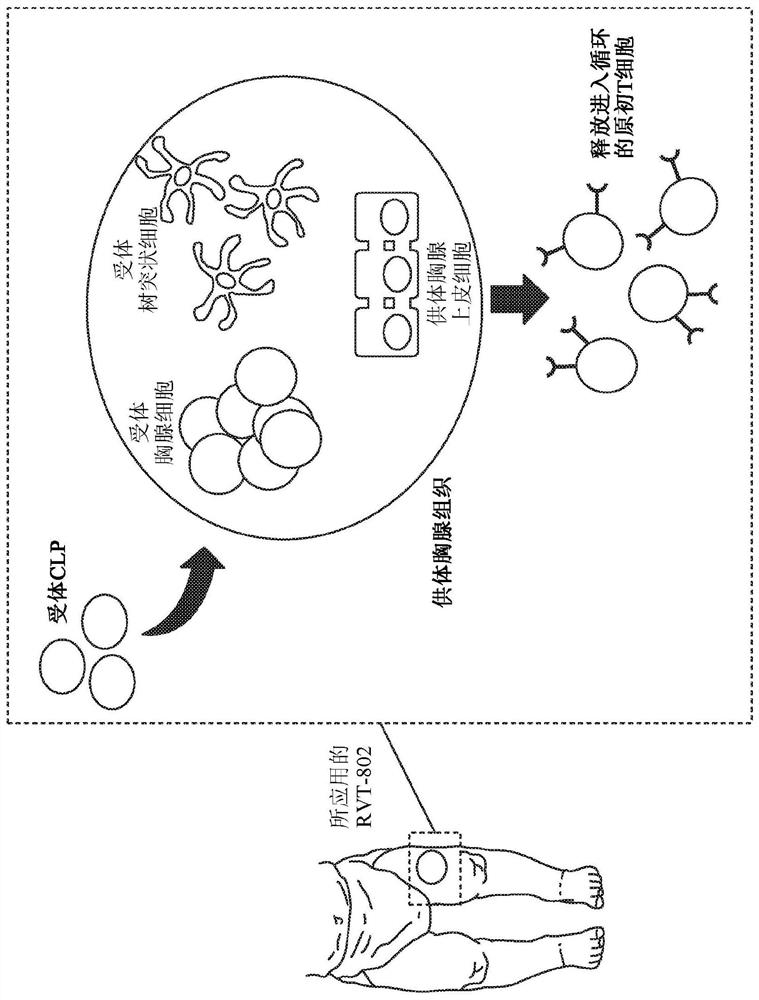
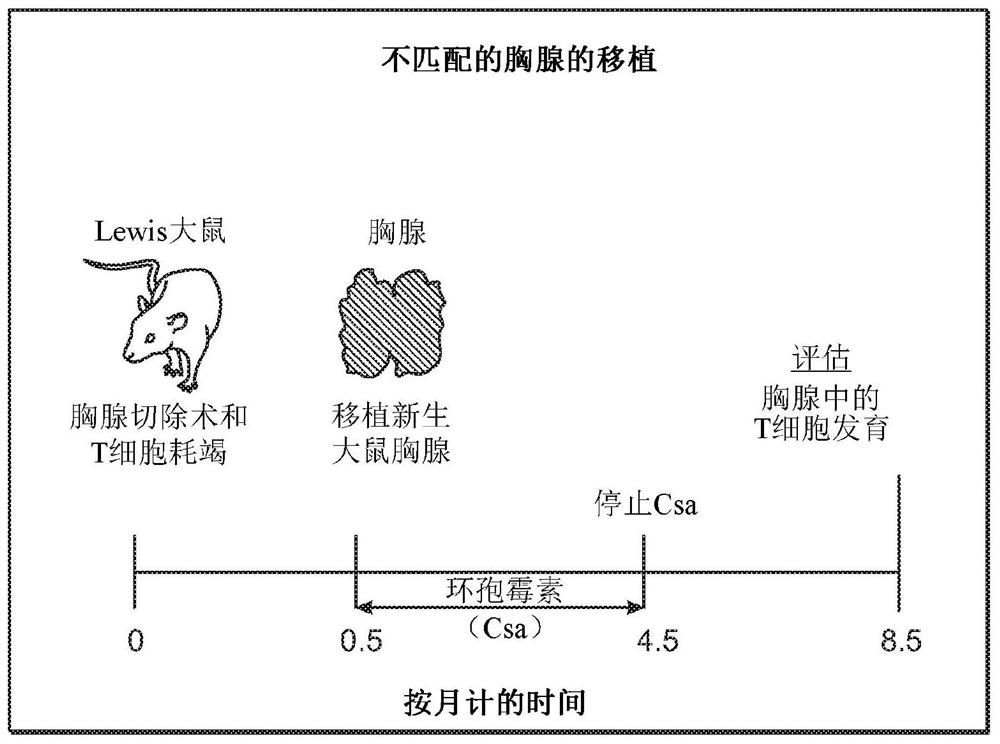
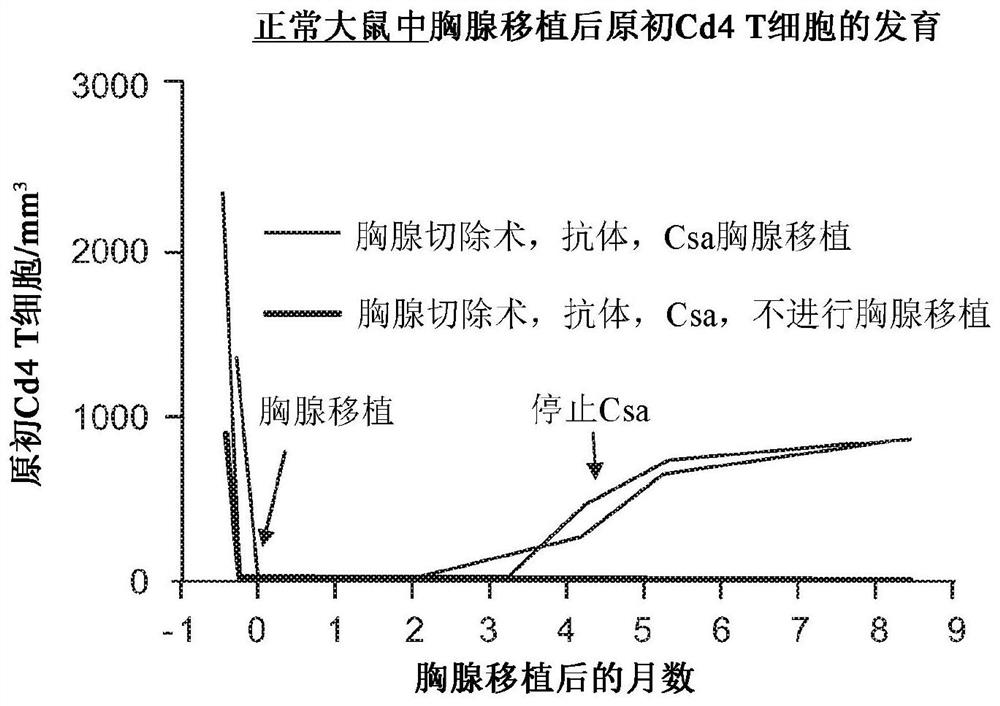
Cultured thymus tissue transplantation promotes donor-specific tolerance to allogeneic solid organ transplants
An allogeneic, tolerant technology that can be used in cell culture supports/coatings, tissue culture, microbial assays/tests, etc., and can solve problems such as induction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0603] Example 1: Study of Intrathymic Variability.
[0604] Intrathymic variability was investigated to determine whether histological test results from one part of the thymus could be considered representative of histological test results from any other part of the same thymus. The results of this test are used to determine how many samples should be tested during routine approval release testing and process validation testing.
[0605]Histological acceptance criteria were established, as previously described, and included assessment of: areas positive for keratin AE1 / AE3 scattered throughout the tissue on days 5-9; identification of at least 1 Herb body ; CK14 staining was scattered throughout the tissue; and intact nuclei were observed.
[0606] For this study, three thymus slices were sliced in a directional fashion, and the position of the slice within each thymus was tracked. Culture slices in 6-well plates to track each slice. Such as Figure 5A Slice as shown. ...
example 2
[0613] Example 2: Time course study of the whole thymus.
[0614] For this study, five thymus were sectioned and cultured following SOP. On the day of sectioning, prepare the first, middle, and final sections for immunohistochemistry. The remainder of each thymus was sectioned and cultured in 6-well plates. Each thymus was applied to one of the following time points: Baseline (Day 0), Day 5, Day 9, Day 12, and Day 21. see Figure 7 Thymus slices on day 0 in, Figure 8 Day 5 slices in, Figure 9 Day 12 slices in and Figure 10 Day 21 slices.
[0615] The total number of slices from each thymus ranged from 21 to 62 slices. Slices were cultured following the procedure described above, changing the medium daily. Submit the sections to the pathology laboratory for H&E staining and analysis for identity, potency and viability. All slides in this study met the acceptance criteria for histological testing for approval release, namely: areas positive for keratin AE1 / AE3 disper...
example 3
[0621] Example 3: Forced degradation studies of thymus tissue.
[0622] In this study, thymus tissue sections were processed to produce tissue sections considered degraded or nonviable. Three thymus were used for these experiments. Control samples were obtained from each thymus. The treatment conditions given in Table 7 were tested.
[0623] Table 7: Forced degradation treatment conditions
[0624]
[0625] Put the 10cm Petri dish containing the slices into a Ziploc bag and place it in a 55°C water bath to complete the thermal shock. The plate is placed on a support and not submerged. Freezing / thawing was accomplished by placing the 10 cm dish in a -20 °C freezer for 4 h and then thawing at ambient.
[0626] Samples were tested for histology on days 5 and 9 of culture. Some samples were also tested on day 21. All slides in this study met the acceptance criteria for histological testing for approval release, namely: areas positive for keratin AE1 / AE3 dispersed through...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com