High-molecular weight Cordyceps militaris polysaccharide, its preparation method and its application in the preparation of anti-complement drugs
A technology of Cordyceps militaris polysaccharide and weight-average molecular weight is applied in directions such as pharmaceutical combinations, medical preparations containing active ingredients, pharmaceutical formulations, etc., to achieve the effect of significant complement inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
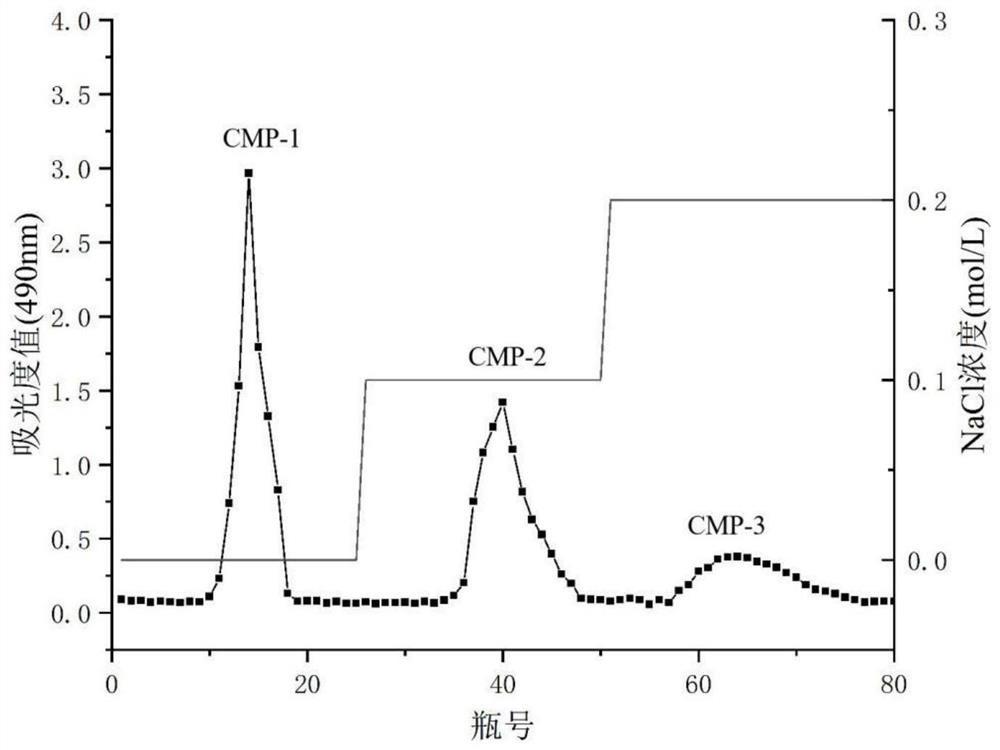
[0026] Example 1 Preparation of Cordyceps militaris polysaccharide CMP-1, CMP-2 and CMP-3
[0027] Take 2.5 kg of Cordyceps militaris medicinal material and pulverize it, extract three times with 95% ethanol, filter to get the dregs, and dry to constant weight. Weigh the dried Cordyceps militaris residue, extract at a liquid-to-solid ratio of 35:1 (mL / g) at an extraction temperature of 90°C for 2 hours, and filter to obtain the filtrate. The filtrate was concentrated to 1 / 5 of the original volume, and 95% ethanol was added to a final concentration of 85%. After standing at 4°C for 24 hours, the supernatant was removed by centrifugation, and the precipitate was dried to constant weight to obtain the crude polysaccharide of Cordyceps militaris. Wet column packing is used, the pretreated DEAE-52 cellulose is loaded into the chromatography column, the crude polysaccharide of Cordyceps militaris is redissolved with deionized water, and the sample is loaded, followed by deionized wa...
Embodiment 2
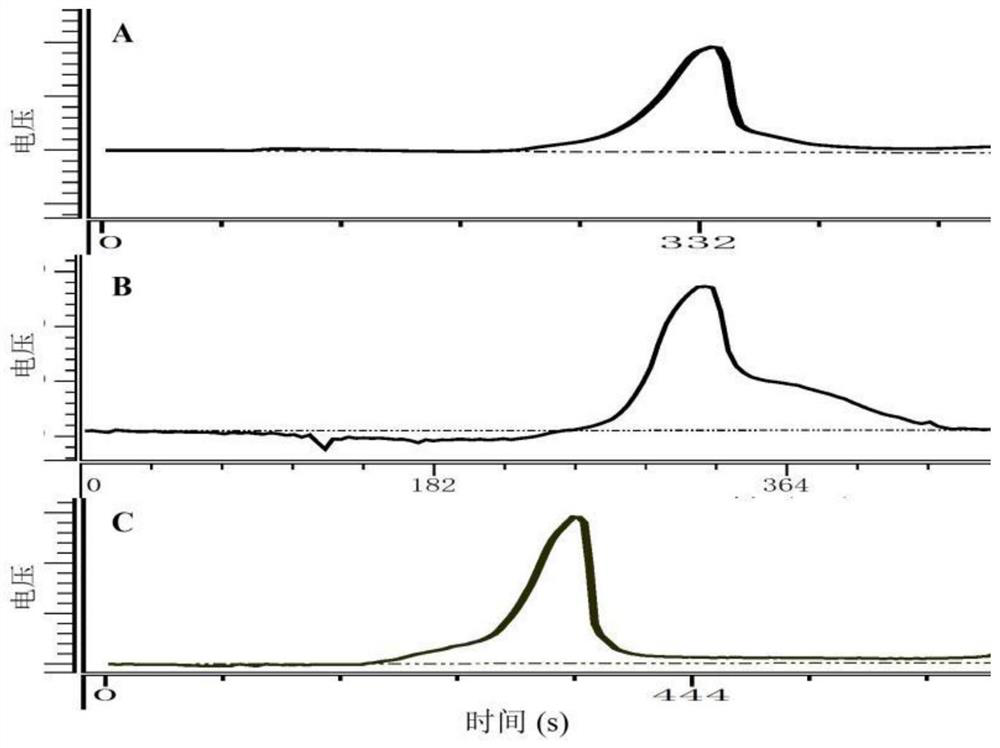
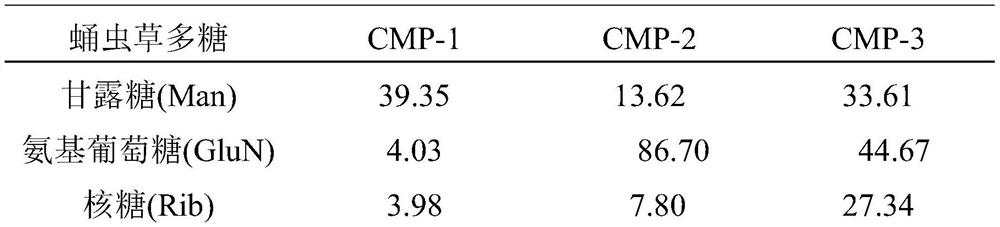
[0028] Structural characterization of embodiment 2 Cordyceps militaris polysaccharide (CMP-1, CMP-2 and CMP-3)
[0029] (1) Determination of weight average molecular weight
[0030]The homogeneity and molecular weight of polysaccharides from Cordyceps militaris were determined by high performance gel permeation chromatography with refractive index detector (HPGPC-RID). Pass 2.0 mg / mL 20 μL of Cordyceps militaris polysaccharide solution through a 0.45 μm microporous membrane, and then inject it into a Shodexsugar KS-804 sugar column (8.0 mm×300 mm). The chromatographic conditions are: ultrapure water is the mobile phase, the flow rate is 1.0 mL / min, the column temperature is 50°C, and the temperature of the differential refraction detector is 35°C. Data processing was recorded on a N2000 GPC chromatography workstation. A series of dextran with different weight-average molecular weights were used as standard products to make a calibration curve to calculate the molecular weigh...
Embodiment 4
[0050] Example 4 Alternative Pathway Complement Inhibition Test
[0051] Normal healthy human serum by GVB-Mg 2+ / EGTA buffer was incubated on ice for 15 min as the source of complement for this bypass pathway. Take 2% rabbit erythrocytes with GVB-Mg 2+ / EGTA buffer to 5×10 8 After cells / mL, NHSP and different concentrations of diluted Cordyceps militaris polysaccharides were added, pre-incubated for 15 min, rabbit red blood cells (Erab) were added and incubated in a 37°C water bath for 30 min, cooled on ice, terminated, and centrifuged. The supernatant of each tube was diluted in a 96-well plate, and the absorbance value was measured at 412nm. At the same time, the blank group, the full hemolysis group, the NHSP group, the negative drug control group (glucose) and the positive drug control group (heparin) were set up in the experiment. . Calculate the hemolysis inhibition rate of Cordyceps militaris polysaccharide under different concentration gradients. And use GraphPad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com