High-molecular-weight cordyceps militaris polysaccharide, preparation method thereof and application of high-molecular-weight cordyceps militaris polysaccharide in preparation of anticomplement drugs
A technology of Cordyceps militaris polysaccharide and weight average molecular weight, which is applied in the direction of drug combination, medical preparations containing active ingredients, and pharmaceutical formulas, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
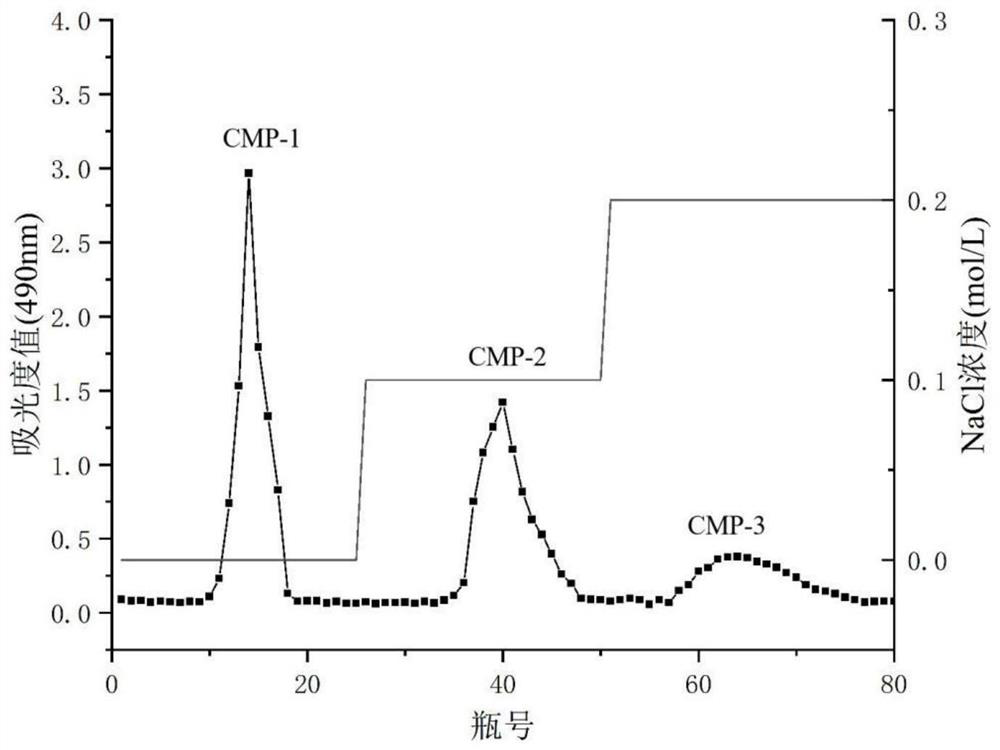
[0026] Embodiment 1 prepares Cordyceps militaris polysaccharide CMP-1, CMP-2 and CMP-3
[0027] Take 2.5kg of Cordyceps militaris material and crush, extract three times with 95% ethanol, filter to get the medicinal residue, and dry to constant weight. Weigh the dried Cordyceps militaris slag, take the liquid-solid ratio of 35:1 (mL / g), extract the temperature at 90° C. for 2 hours, and filter to obtain the filtrate. The filtrate was concentrated to 1 / 5 of the original volume, and 95% ethanol was added to the final concentration of 85%. After standing at 4°C for 24 hours, the supernatant was removed by centrifugation, and the precipitate was taken and dried to constant weight to obtain Cordyceps militaris crude polysaccharide. Using wet packing, the pretreated DEAE-52 cellulose was loaded into the chromatography column, the crude polysaccharide of Cordyceps militaris was redissolved in deionized water, and the samples were loaded, followed by deionized water, 0.1mol / L and 0.2m...
Embodiment 2
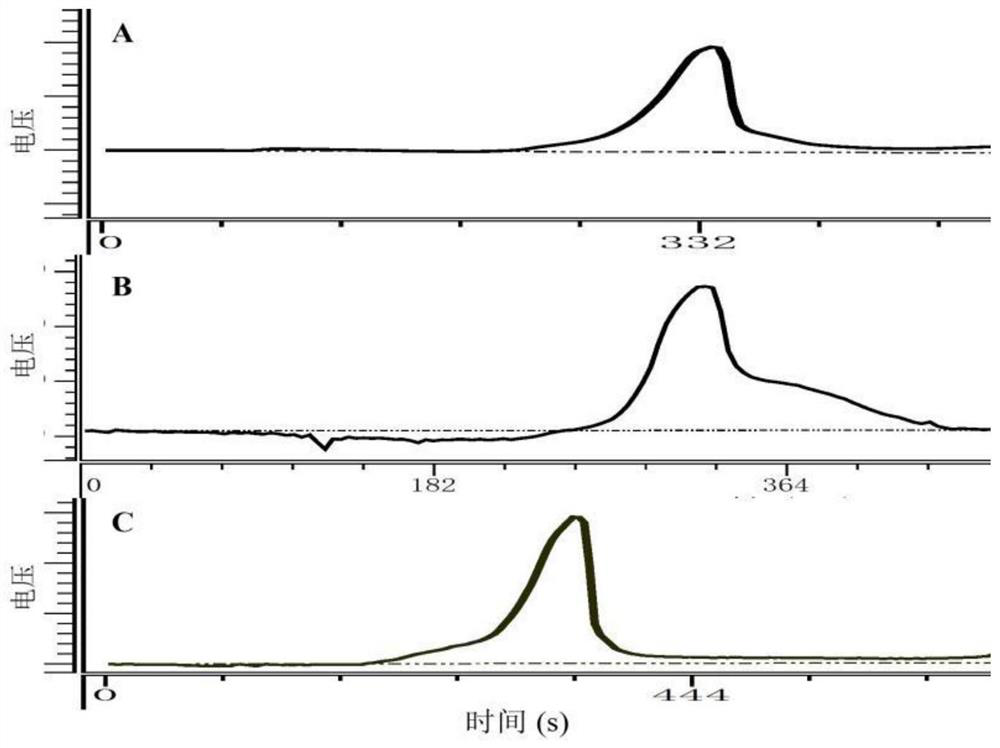
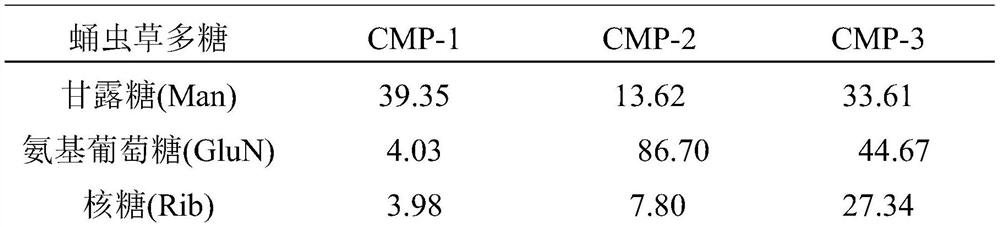
[0028] Embodiment 2 The structural characterization of Cordyceps militaris polysaccharides (CMP-1, CMP-2 and CMP-3)
[0029] (1) Determination of weight average molecular weight
[0030]The homogeneity and molecular weight of Cordyceps militaris polysaccharides were determined by high performance gel permeation chromatography combined with refractive index detector (HPGPC-RID). The 2.0 mg / mL 20 μL Cordyceps militaris polysaccharide solution was passed through a 0.45 μm microporous membrane, and then injected into a Shodexsugar KS-804 sugar column (8.0 mm×300 mm). The chromatographic conditions were as follows: ultrapure water was the mobile phase, the flow rate was 1.0 mL / min, the column temperature was 50°C, and the temperature of the differential refraction detector was 35°C. Data processing was recorded on an N2000 GPC chromatography workstation. The molecular weights of polysaccharides were calculated using a series of dextran with different weight average molecular weig...
Embodiment 4
[0050] Example 4 Alternative pathway complement inhibition test
[0051] Normal healthy human serum by GVB-Mg 2+ / EGTA buffer was incubated on ice for 15min as the source of complement for this alternative pathway. Take 2% rabbit erythrocytes with GVB-Mg 2+ / EGTA buffer to 5×10 8 After cells / mL, NHSP and different concentrations of Cordyceps militaris polysaccharide after dilution were added, pre-incubated for 15 min, and rabbit erythrocytes (Erab) were added and incubated in a 37°C water bath for 30 min, cooled on ice, terminated the reaction, and centrifuged. Dilute the supernatant from each tube in a 96-well plate, and measure the absorbance at 412 nm. At the same time, a blank group, a complete hemolysis group, an NHSP group, a negative drug control group (glucose) and a positive drug control group (heparin) were set up in the experiment. . The inhibition rate of hemolysis under different concentration gradients of Cordyceps militaris polysaccharide was calculated. An...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com