A metal-air battery based on fe(ii)/fe(iii) chemical oxidation and electrochemical reduction
A metal-air battery and chemical oxidation technology, which is applied in the direction of fuel cell half-cells and secondary battery-type half-cells, can solve problems such as loss of activity, pollute the environment, and complicate preparation, and achieve the effect of increasing the discharge voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
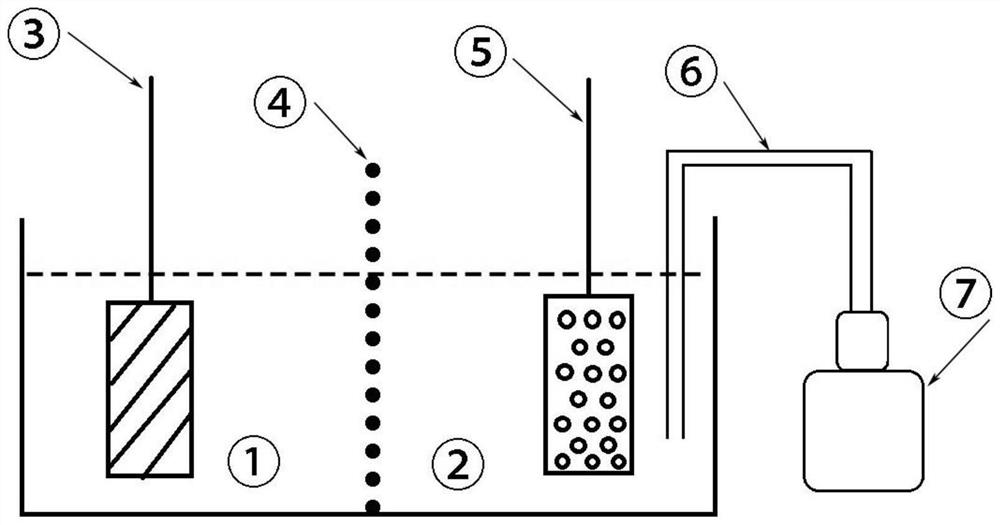
[0043] Immerse the industrial pure aluminum metal plate in the degassed saturated sodium chloride solution to obtain the negative electrode of the battery;
[0044] Immerse the graphite felt electrode in 0.5mol / L acid ferric nitrate solution to obtain the positive electrode of the battery;
[0045] The negative electrode electrolyte and the positive electrode electrolyte are separated by an anion semi-permeable membrane;
[0046] One end of the vent tube is immersed in the positive electrolyte, and the other end is connected to an air pump to form a metal-air battery based on Fe(II) / Fe(III) chemical oxidation and electrochemical reduction.
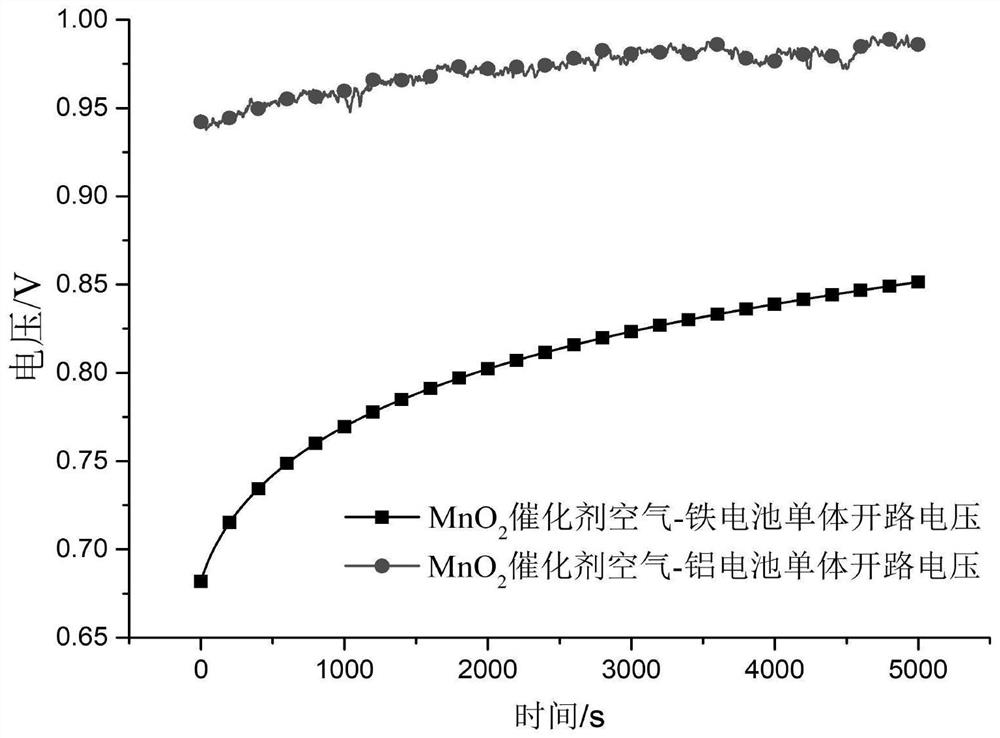
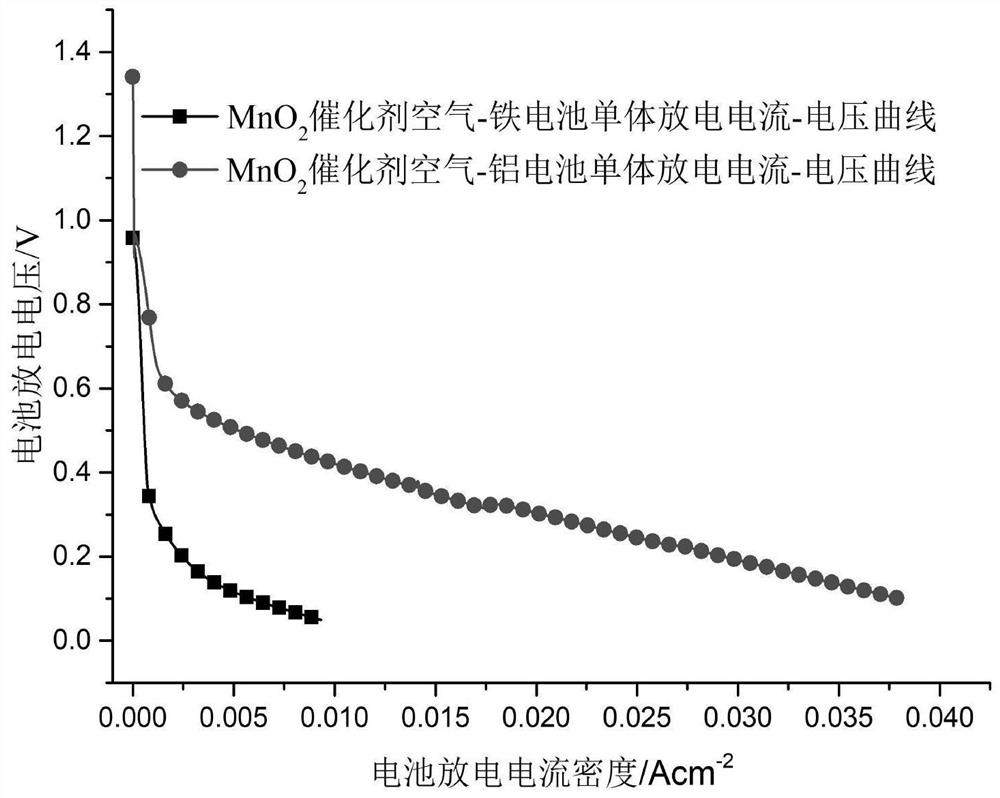
[0047] Test the discharge performance of the battery, the open circuit voltage is 1.33V, and the maximum discharge current density is 70mA×cm -2 .
Embodiment 2
[0049] Immerse the industrial pure aluminum metal plate in the degassed 3mol / L sodium chloride solution to obtain the negative electrode of the battery;
[0050] Immerse the graphite felt electrode in 1mol / L acid ferric sulfate solution to obtain the positive electrode of the battery;
[0051] The negative electrode electrolyte and the positive electrode electrolyte are separated by an anion semi-permeable membrane;
[0052] One end of the vent tube is immersed in the positive electrolyte, and the other end is connected to an air pump to form a metal-air battery based on Fe(II) / Fe(III) chemical oxidation and electrochemical reduction.
[0053] Test the discharge performance of the battery, the open circuit voltage is 1.41V, and the maximum discharge current density is 80mA×cm -2 .
Embodiment 3
[0055] Immerse the industrial pure aluminum metal plate in the degassed 2mol / L sodium chloride solution to obtain the negative electrode of the battery;
[0056] Immerse the graphite felt electrode in 2mol / L acid ferric chloride solution to obtain the positive electrode of the battery;
[0057] The negative electrode electrolyte and the positive electrode electrolyte are separated by an anion semi-permeable membrane;
[0058] One end of the vent tube is immersed in the positive electrolyte, and the other end is connected to an air pump to form a metal-air battery based on Fe(II) / Fe(III) chemical oxidation and electrochemical reduction.
[0059] Test the discharge performance of the battery, the open circuit voltage is 1.41V, and the maximum discharge current density is 80mA×cm -2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com