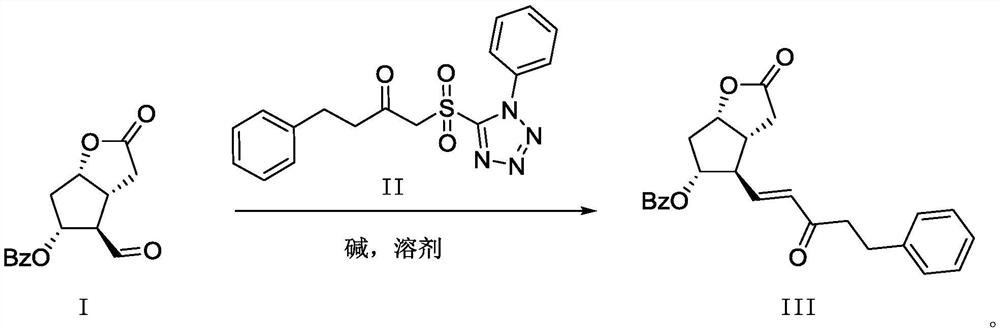
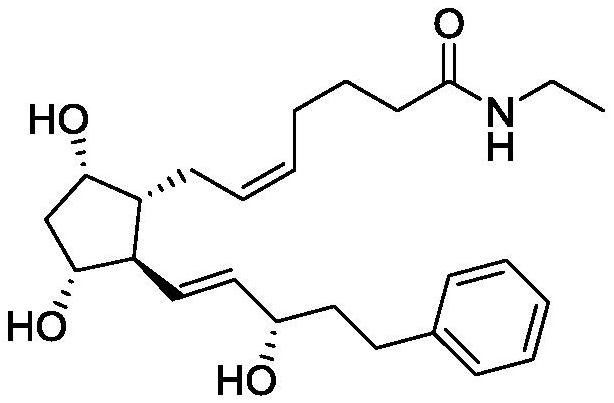
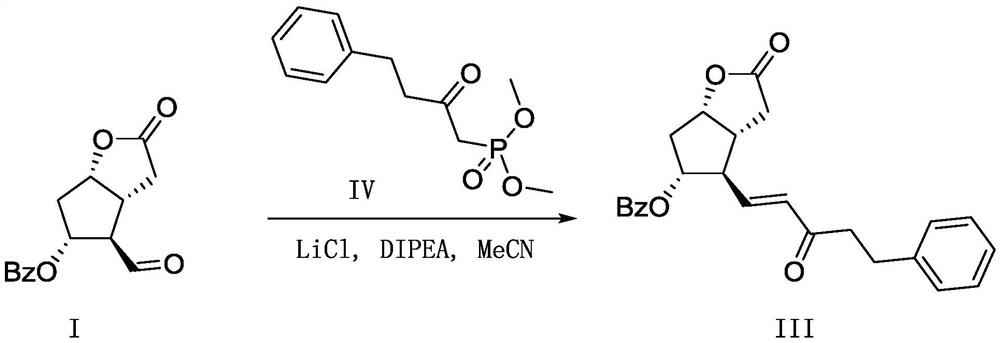
A kind of preparation method of bimatoprost pharmaceutical intermediate
A bimeteriprost and intermediate technology is applied in the field of synthesis of bimeteriprost pharmaceutical intermediates, and achieves the effects of high stereoselectivity, easy acquisition of raw materials, and easy industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023] Pre-prepared solution: under the protection of nitrogen, dissolve 2.69g potassium tert-butoxide (0.024mol) in 14g tert-butanol, stir until dissolved and seal it for use. 2.93g (0.0107mol) of compound I and 4.21g (0.0118mol) of compound II were dropped into a 250ml three-necked flask successively, and the nitrogen gas was replaced in vacuum 5 times, and then 130g of DCM (dichloromethane) was injected inwardly under the protection of nitrogen gas, and the temperature was lowered to -60°C.
[0024] Inject all the potassium tert-butoxide / tert-butanol solution prepared above, control the temperature not to exceed -50°C, and finish the injection in about 10 minutes. After the injection, keep it at -60~-55°C for 5-6 hours, and then heat up naturally to room temperature. Transfer the above reaction solution into a 250ml single-necked bottle and spin dry. The spin-dried solid was slurried with 80 g of water at room temperature for 2 hours, and filtered. The solid ...
Embodiment 2
[0026]
[0027] Pre-prepared solution: under the protection of nitrogen, dissolve 5.38g potassium tert-butoxide (0.048mol) in 14g tert-butanol, stir until dissolved and seal it for use. 2.93 g (0.0107 mol) of compound I and 8.42 g (0.0118 mol) of compound II were sequentially put into a 250 ml three-necked flask, replaced by nitrogen vacuum for 5 times, and then 130 g of DCM was injected inward under the protection of nitrogen, and the temperature was lowered to -60°C with stirring.
[0028] Inject all the potassium tert-butoxide / tert-butanol solution prepared above, control the temperature not to exceed -50°C, and finish the injection in about 10 minutes. After the injection, keep it at -60~-55°C for 5-6 hours, and then heat up naturally to room temperature. Transfer the above reaction solution into a 250ml single-necked bottle and spin dry. The spin-dried solid was slurried with 80 g of water at room temperature for 2 hours, and filtered. The solid was slurried with 80 ...
Embodiment 3
[0030]
[0031] Pre-prepared solution: under the protection of nitrogen, dissolve 2.31g sodium tert-butoxide (0.024mol) in 14g tert-butanol, stir until dissolved and seal it for use. 2.93g (0.0107mol) of compound I and 4.21g (0.0118mol) of compound II were dropped into a 250ml three-necked flask successively, and the nitrogen gas was replaced in vacuum 5 times, and then 130g of DCM (dichloromethane) was injected inwardly under the protection of nitrogen gas, and the temperature was lowered to -60°C.
[0032] Inject all the potassium tert-butoxide / tert-butanol solution prepared above, control the temperature not to exceed -50°C, and finish the injection in about 10 minutes. After the injection, keep it at -60~-55°C for 5-6 hours, and then heat up naturally to room temperature. Transfer the above reaction solution into a 250ml single-necked bottle and spin dry. The spin-dried solid was slurried with 80 g of water at room temperature for 2 hours, and filtered. The solid was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com