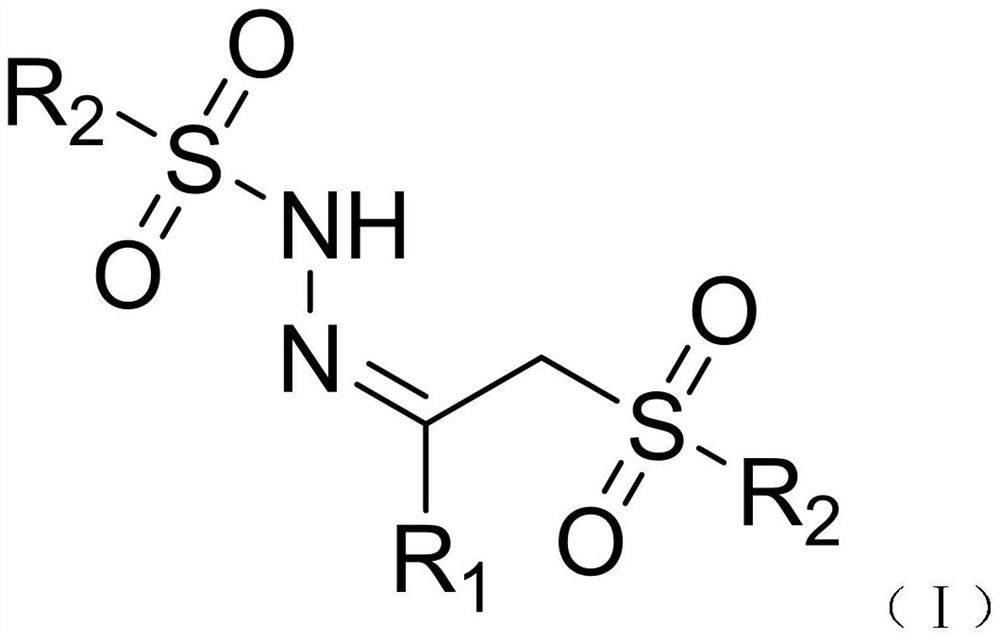
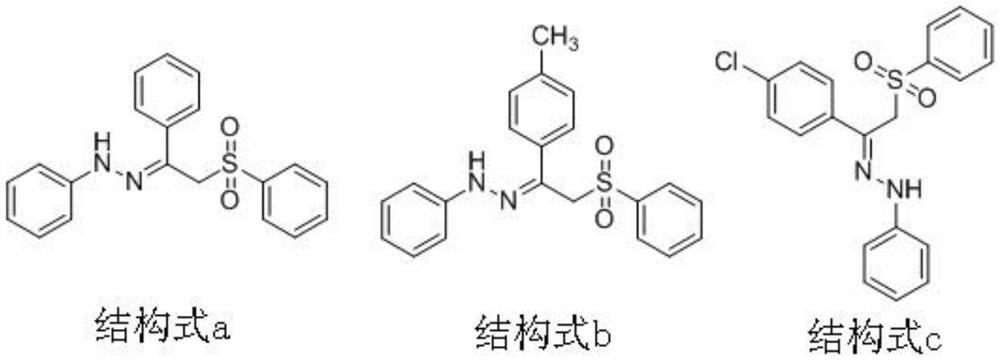
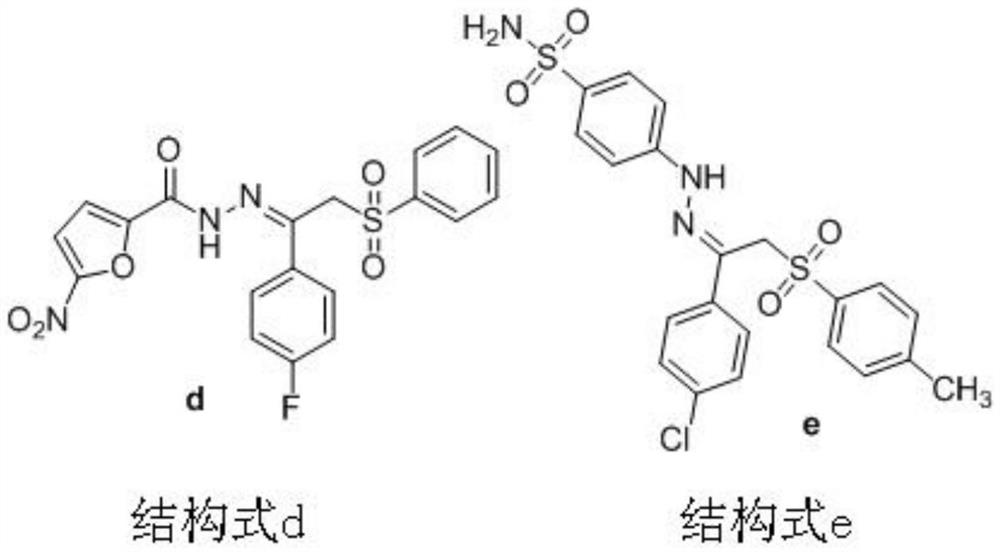
Beta-sulfonyl hydrazone derivative and preparation method and application thereof
A technology of derivatives and sulfone hydrazone, which is applied in the field of β-sulfone hydrazone derivatives and its preparation, can solve the problems of low adaptability and low yield of the group, and achieve good reaction effect and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~11
[0042] The solvent of Examples 1-11 is ethanol, and different catalysts are used for the reaction, and the reaction results are shown in Table 1.
[0043] The reaction conditions and the result of table 1 embodiment 1~11
[0044]
Embodiment 12~20
[0046] The catalyzer of embodiment 12~17 adopts CuSO4.5H 2 O, the results of changing the reaction solvent are shown in Table 2.
[0047] The reaction conditions and the result of table 2 embodiment 12~17
[0048]
[0049]
[0050] The catalyzer of embodiment 18~20 adopts CuSO4.5H 2 O, the reaction solvent is water, and the results of changing the reaction temperature are shown in Table 3.
[0051] The influence of table 3 different reaction temperature on reaction
[0052]
Embodiment 21~41
[0054] The catalyzer of embodiment 21~41 adopts CuSO4.5H 2 O, water was used as the solvent, and the substrate was changed. The results obtained are shown in Table 4.
[0055] The reaction conditions and the result of table 4 embodiment 21~41
[0056]
[0057]
[0058] The structural characterization data of some products are as follows:
[0059]
[0060] N'-(1-Phenyl-2-(phenylsulfonyl)ethylene)benzenesulfonohydrazide(21):White solid,m.p:94.5-95.5℃. 1 H NMR (400MHz, CDCl 3 )δ9.59(s,1H),8.00(d,J=8.2Hz,2H),7.95-7.92(m,1H),7.90-7.88(m,1H),7.81–7.77(m,1H),7.73 –7.71(m,2H),7.68(d,J=8.2Hz,2H),7.65-7.61(m,3H),7.40-7.37(m,3H),4.55(s,2H); 13 C NMR (100MHz, CDCl 3 )δ155.41,138.31,137.60,135.15,134.79,133.43,130.21,129.55,129.08,128.44,128.32,128.15,126.41,55.80.HRMS(ESI)M / Z calcd for C 20 h 19 N 2 o 4 S 2 .[M+H] + :415.0781.Found:415.0784.
[0061]
[0062] 4-Methoxy-N'-(2-((4-methoxyphenyl)sulfonyl)-1-phenylethylidene)benzenesulfonohydrazide(27):White solid,m.p:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com