Heterocyclic compound as well as preparation method and application thereof
A technology of heterocyclic compounds and compounds, applied in organic chemistry, measuring devices, instruments, etc., can solve problems affecting the quality of raw materials and finished products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
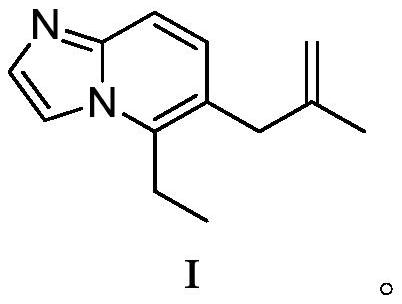
[0087] Embodiment 1: Preparation contains the crude product of compound I:
[0088]
[0089]Take a 5L four-necked flask, blow nitrogen flow into it for 5 minutes, add compound 1 (0.76mol, 150.00g) into the reaction flask, add THF (0.75L), turn on the nitrogen protection, turn on the stirring, and wait until the internal temperature drops to 10 At ~15°C, start to add EtMgBr solution (2.28mol, 1.14L) dropwise. During the dropping process, keep the internal temperature below 20°C. After the dropwise addition, start heating, control the internal temperature at 60-65°C, and react for 1.5-2.0 hours , turn on the cooling, and when the internal temperature drops to 10-15°C, start to add 3-chloro-2-methylpropene (3.04mol, 275g) dropwise, keep the internal temperature below 40°C during the dropwise addition, within 20-30 minutes After the dropwise addition is completed, turn on the heating device and maintain the reaction at 60-65° C. for 2 hours. After the reaction is complete, tur...
Embodiment 2
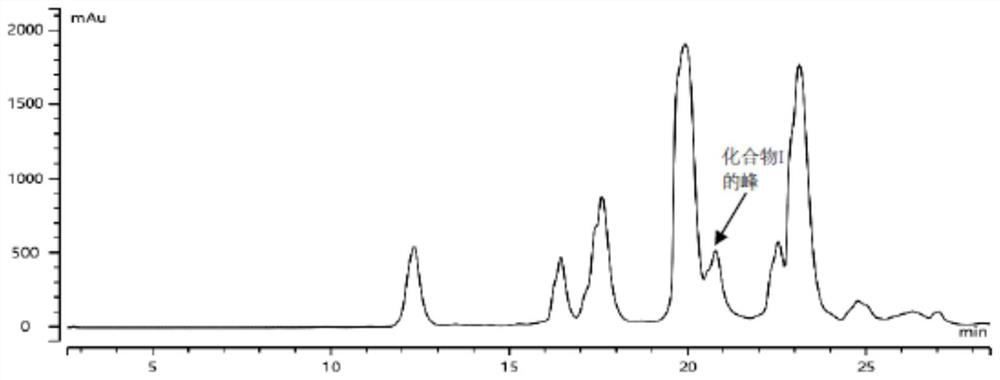
[0090] Embodiment 2: the preparation separation and purification method of compound I
[0091] Step 1: Solution preparation of intermediate 2 crude product
[0092] Take the crude product of intermediate 2 in Example 1, add tetrahydrofuran, deionized water and acetonitrile (about 2:1:1 ratio) and dissolve to 20mg / ml, use a syringe filter (nylon NY 0.45μm-25mm) to filter and set aside.
[0093] Step 2: Optimization of the solution separation and purification method for the crude intermediate 2
[0094] The separation degree of the solution of the crude product of intermediate 2 was investigated respectively under neutral, acidic and alkaline conditions, wherein: under neutral conditions, mobile phase A was purified water; under acidic conditions, mobile phase A was purified water containing 0.1% trifluoro Purified water of acetic acid; under alkaline conditions, mobile phase A is purified water containing 0.1% ammonia 10mM ammonium bicarbonate;
[0095] Other analysis conditi...
Embodiment 3
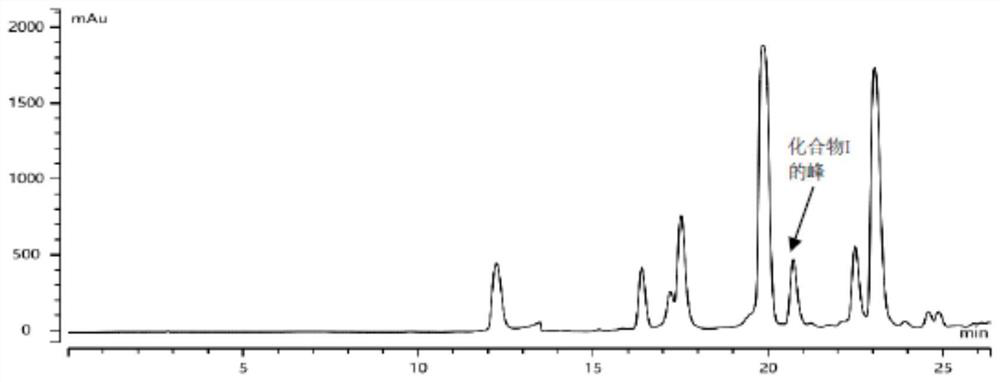
[0114] Embodiment 3: Compound 1 is used for the quality control detection of intermediate 2
[0115] Take an appropriate amount of intermediate 2 crude product prepared in Example 1, add methanol to dissolve and dilute to make a solution containing about 1 mg per 1 ml, as the test solution; in addition, take an appropriate amount of Compound I reference substance prepared in Example 2, add methanol Dissolve and dilute to make a solution containing about 2 μg per 1 ml, as the reference solution. According to high performance liquid chromatography (Chinese Pharmacopoeia 2015 edition four general rules 0512), with octadecylsilane bonded silica gel as filler (YMC-Triart C 18 , 4.6×250mm, 5μm, or other chromatographic columns with equivalent performance); use 10mmol / L dipotassium hydrogen phosphate solution (adjust the pH value to 7.5 with phosphoric acid) aqueous solution-acetonitrile (95:5) as mobile phase A, and use 10mmol / L L dipotassium hydrogen phosphate solution (adjust the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com