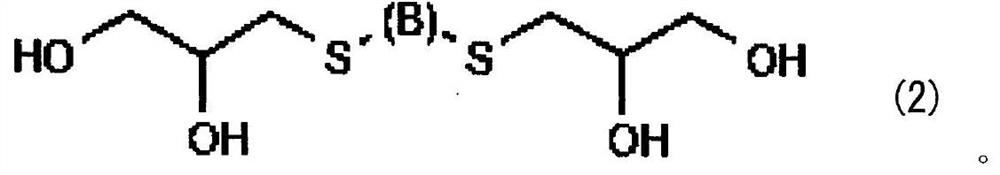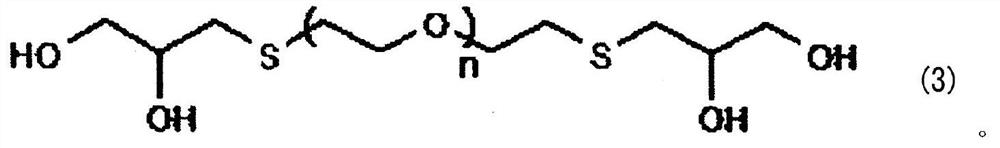Tin alloy plating solution
A technology of tin alloy and plating solution, applied in the direction of organic chemistry, etc., can solve the problems of easy decomposition and precipitation of silver, etc., to achieve good water solubility, good appearance and uniformity of film thickness, excellent electrolytic stability and stability over time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 Embodiment approach >
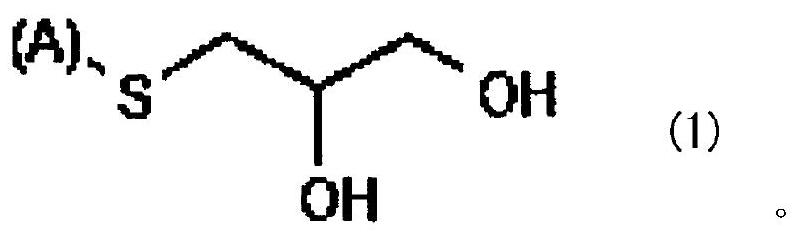
[0044] The tin alloy plating solution of the first embodiment contains a soluble tin salt, a soluble salt of a metal less oxidizable than tin, and a sulfur compound represented by the above general formula (1). This tin alloy plating solution may further contain additives.
[0045] 〔Soluble tin salt〕
[0046] The soluble tin salt used in the tin alloy plating solution of the first embodiment is a salt that dissolves in water and generates divalent tin ions. Examples of soluble tin salts include halides, sulfates, oxides, alkanesulfonates, arylsulfonates and alkanolsulfonates. Specific examples of alkanesulfonate include methanesulfonate and ethanesulfonate. Specific examples of arylsulfonate include benzenesulfonate, phenolsulfonate, cresolsulfonate and toluenesulfonate. Specific examples of alkanol sulfonates include isethionates.
[0047] The soluble tin salt may be used alone or in combination of two or more. The content of the soluble tin salt in the tin alloy plating...
no. 2 Embodiment approach >
[0077] The tin alloy plating solution of the second embodiment contains a soluble tin salt, a soluble salt of a metal less oxidizable than tin, and a sulfur compound represented by the general formula (2). This tin alloy plating solution may further contain additives.
[0078] The soluble tin salt contained in the tin alloy plating solution of the second embodiment, the soluble salt of a metal less oxidizable than tin, and the additive are the same as the soluble tin salt contained in the tin alloy plating solution of the first embodiment, the soluble salt less oxidizable than tin The metal soluble salts and additives are the same, so repeated explanations are omitted.
[0079] [Sulfur compound represented by general formula (2)]
[0080] The sulfur compound used in the tin alloy plating solution of the second embodiment is represented by the above general formula (2), and functions as a complexing agent for metals less oxidizable than tin. After mixing α-thioglycerol as the...
no. 3 Embodiment approach >
[0083] The tin alloy plating solution of the third embodiment contains a soluble tin salt, a soluble salt of a metal less oxidizable than tin, and a sulfur compound represented by the general formula (3). This tin alloy plating solution may further contain additives.
[0084] The soluble tin salt contained in the tin alloy plating solution of the third embodiment, the soluble salt of a metal less oxidizable than tin, and the additive are the same as the soluble tin salt contained in the tin alloy plating solution of the first embodiment, and the soluble salt of a metal less oxidizable than tin. The metal soluble salts and additives are the same, so repeated explanations are omitted.
[0085] [Sulfur compound represented by general formula (3)]
[0086] The sulfur compound used in the tin alloy plating solution of the third embodiment is represented by the above general formula (3), and functions as a complexing agent for metals less oxidizable than tin. After mixing α-thiogl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com