Preparation method of the reference substance of cyanidin-3-coumaroyl-diglucose-5-glucoside
A technology of glucoside and coumaroyl, applied in the field of preparation of bluebonnet-3-coumaroyl-diglucose-5-glucoside, can solve the problem of unmentioned, inability to obtain high-purity products, and inability to provide flower colors Glycosides and other problems, to achieve the effect of simple and easy method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] After extensive and in-depth research, the inventors first developed a method for preparing a high-purity cyanidin-3-coumaroyl-diglucose-5-glucoside reference substance. Separation method of the present invention comprises the following steps: comprises the following steps successively:
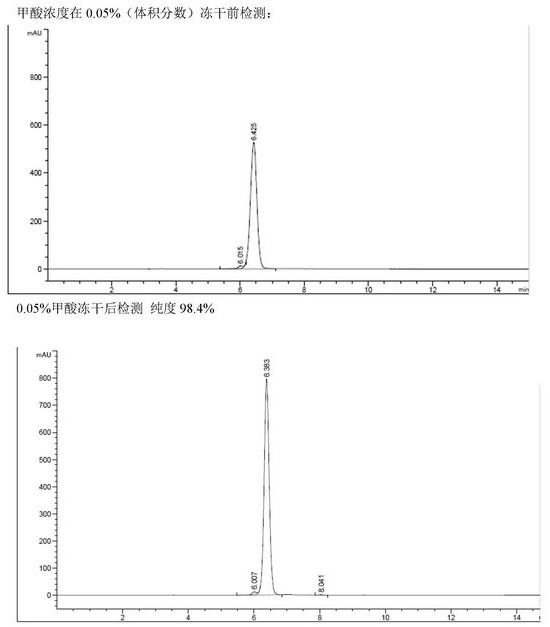
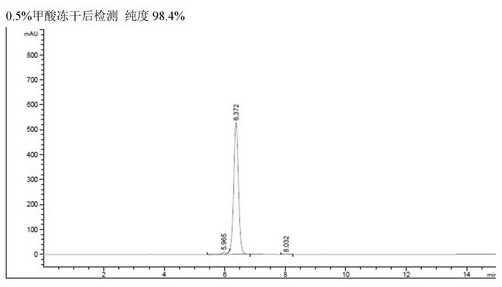
[0037] In the high-pressure reverse-phase chromatography column separation process, the crude product containing cyanidin-3-coumaroyl-diglucose-5-glucoside is loaded onto the high-pressure reverse-phase chromatography column, and the pressure is 5-15.0Mpa to contain 0.05-1 % formic acid and 15%-20% acetonitrile in water to obtain a solution of the target object.
[0038] In the solidification process, the solution of the target substance obtained in the reverse phase chromatography column preparation process is concentrated until the concentration of cyanidin-3-coumaroyl-diglucose-5-glucoside is 5% to 50%, and the concentrated solution is frozen After drying, a solid powder of the forma...
Embodiment 1
[0060] A. Raw material extraction: Add 0.5kg cabbage red powder to 5L methanol solution containing 0.05% hydrochloric acid, ultrasonically extract 3 times, 1 hour each time, cool to room temperature, filter with suction, combine the filtrates, concentrate at 45 degrees until there is no alcohol smell, 500mL;
[0061] B. Macroporous resin enrichment: Weigh 8Kg AB-8 macroporous adsorption resin and load it into a column, use the concentrated solution of step A to dynamically adsorb and load the sample with macroporous resin, then use 20L of water to elute impurities, and then use 30L of 0.05 % hydrochloric acid methanol solution to elute the target substance, collect the eluate, and concentrate to 1L;
[0062] C. Medium-pressure preparation: A medium-pressure column is loaded with a concentrated solution of a crude product with a solid content of about 100g, and the target content is about 10g, and 4 columns are loaded in parallel.
[0063] Medium-pressure preparative column: D...
Embodiment 2
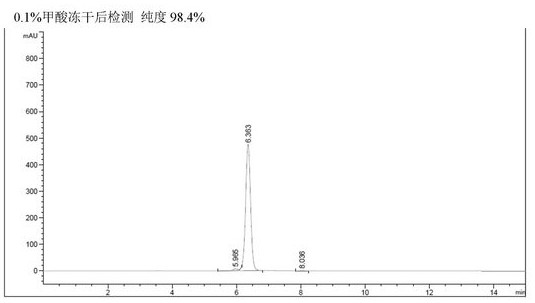
[0071] Except that in the step of E. high pressure preparation, gradient elution was carried out using the eluent of 15%-20% acetonitrile (containing 0.1% formic acid), the rest were performed in the same manner as in Example 1 to obtain cyanidin-3 with a purity of 98.4%. - Coumaroyl-diglucose-5-glucoside about 0.55 g. The HPLC spectrum of the liquid before the solid product recovery process, and the HPLC spectrum of the solid after freeze-drying can refer to figure 2 , can stably provide high-purity formate of the target product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com