Variant SH2 structural domain having high affinity with tyrosine-containing phosphorylation modification peptide
A technology of tyrosine phosphorylation and structural domains, which is applied in the field of variant SH2 domains with high affinity to modified peptides containing tyrosine phosphorylation, which can solve the problems of inability to use and minimize the possibility of immune response Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1. Identification of variant SH2 domains by phage display technology
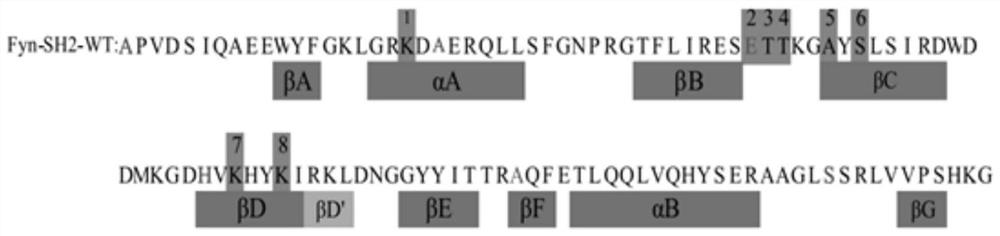
[0072] Amino acid residues at eight positions in the human Fyn SH2 domain were randomly substituted with one of 20 natural amino acids to identify variant SH2 domains that bind pTyr-containing peptides.
[0073] The number of all amino acid residues in the human Fyn SH2 domain is the full-length isoform according to the UniProt database entry (entry) FYN_HUMAN. The gene encoding the wild-type FynSH2 domain between Ala139 and Gly249 (see SEQ ID NO: 1 for its amino acid sequence) was subcloned into pDEST15 vector (Invitrogen Canada Inc.). The three cysteine residues in SEQ ID NO: 1 were replaced with serine residues by QuikChange II Site-Directed Mutagenesis Kit (Qiagen Inc.). This mutation produces the protein sequence shown in SEQ ID NO:2.
[0074] The gene encoding the protein shown in SEQ ID NO: 2 was fused to the gene encoding the major coat protein of M13 bacteriophage. By mutating 8...
Embodiment 2
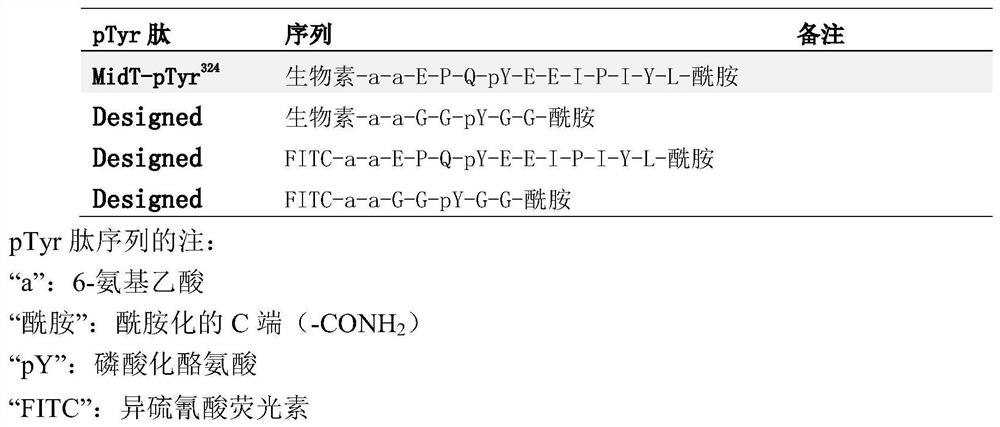
[0077] Example 2, Enhanced binding of variant Fyn SH2 domains to pTyr-containing peptides in vitro
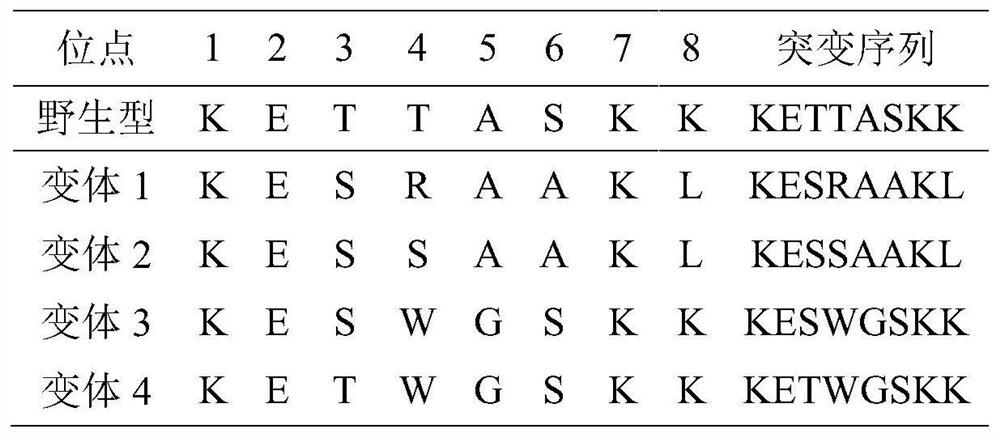
[0078] Single or multiple substitutions were introduced into the wild-type Fyn SH2 domain, and the degree of affinity enhancement resulting from the introduction of these substitutions was determined. Using this wild-type construct as a template, three variant Fyn SH2 domains were constructed by a site-directed mutagenesis approach.
[0079] In the following description, the residue to be substituted is specified using a position number. For example, T8V indicates a substitution of Thr at position 8 to Val in the wild type construct. T4V / S6A represents 2 substitutions in combination, Thr at position 4 to Val and Ser at position 6 to Ala for the wild type construct. The amino acid sequences between positions 1 and 8 of the SH2 domains of the three variants are listed in the sequence listing. By substituting the wild-type construct (SEQ ID NO:1), the following variant Fyn SH2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com