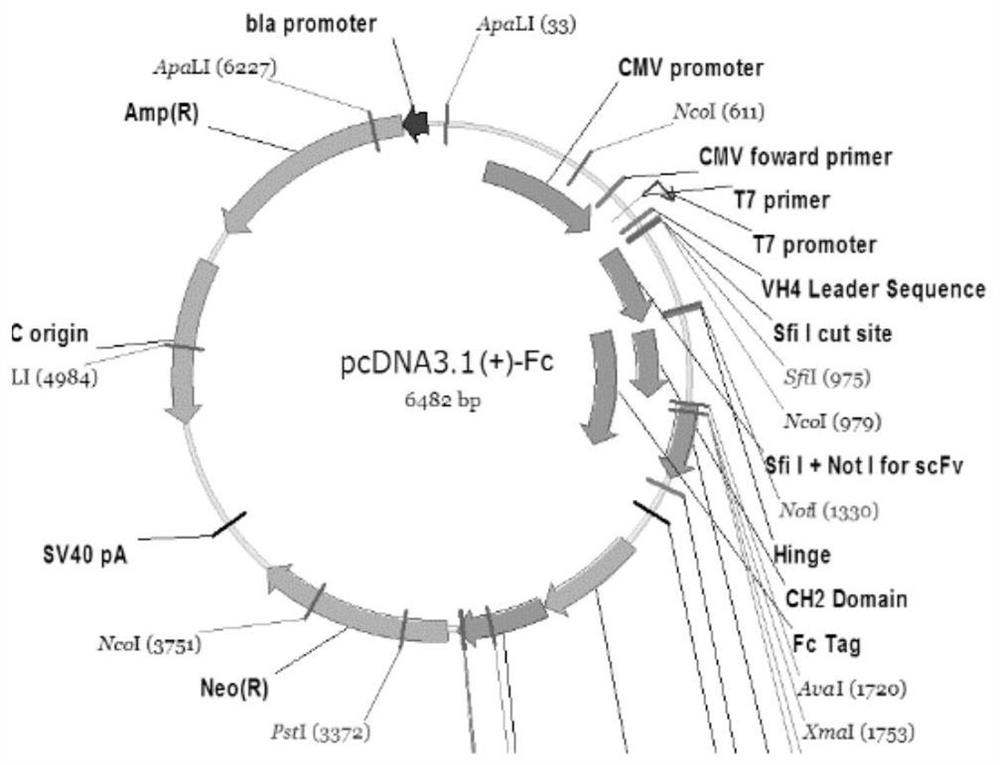
A broad-spectrum neutralizing antibody against HIV
An antibody and carrier technology, applied in the field of cellular immunology and genetic engineering, can solve the problems of virus mutation and poor immunogenicity, and achieve high success rate and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1 Preparation of an anti-HIV antibody
[0068] 1, sorting of mature B cells
[0069] Buffer1: 500ml 1 × PBS + 2mL 0.5m EDTA + 25ml 10% BSA (including 2mm EDTA, 0.5% BSA)
[0070] (1) Collect people from blood of 200 μl of blood, 200 μl of BUFFER1;
[0071] (2) Add 10x volumes of Ack Lysing Buffer (Fisher / Biowhittaker), 10 min, 1500 rpm for 10min, 1500 rpm;
[0072] (3) Abandon the discard, add 10ml buffer 1 washing cells, 1500 rpm centrifuge for 10 min;
[0073] (4) Abandon the discard, add 10 μl of Buffer 1 resuscitation; add the following flow antibody, labeled mature B cells:
[0074] Anti-CD5 / Fitc (UCHT2, EBISCIENCE)
[0075] Anti-CD19 / PE (SJ25-C1, BD Pharmingen)
[0076] Anti-CD10 / APC (BC96, EBIoscience)
[0077] Anti-CD27 / PE-CY7 (O323, EBIoscience)
[0078] Anti-IgM / PE-CY5 (G20-127, BD Pharmingen)
[0079] (5) FACS SORTER sorted mature B cells (CD5-CD19 + CD10-CD27-IgM +), collected a single cell into a 96-well PCR plate, built-in 4 μl of cell lysa...
Embodiment 2
[0097] Example 2 Detection of antibody characteristics
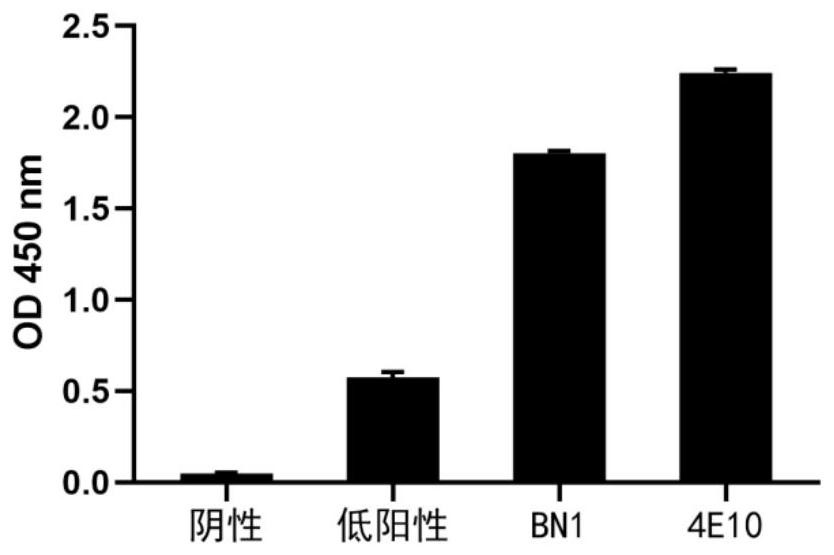
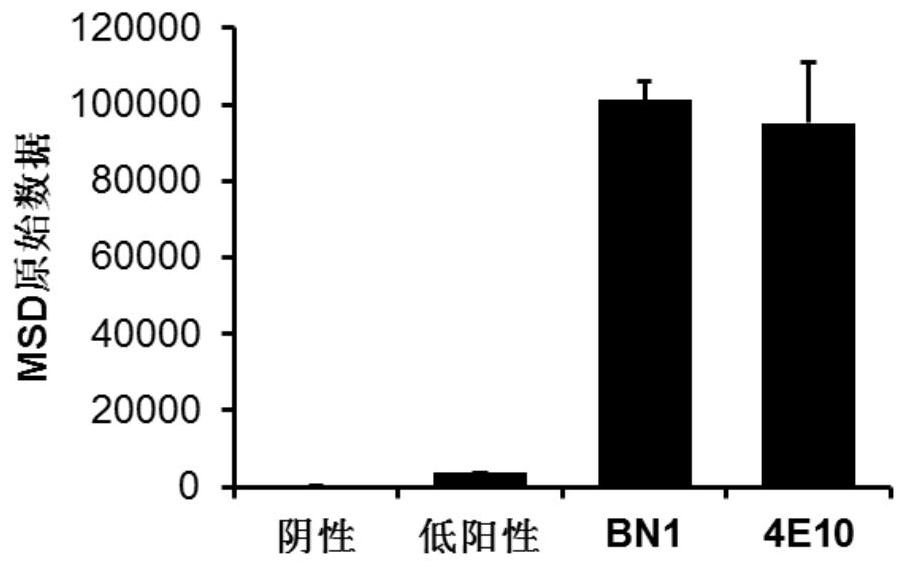
[0098] 1, self-reactive detection
[0099] Antibody self-reactivity adopts clinical standard antinear antibody detection kit (Quanta Lite TM Anaelisa, Inova Diagnostics, Inc.) Perform a negative reaction sample, a low-positive reaction sample, a high-positive reaction sample in the detection kit. 4E10 is an HIV broad spectrum and antibody, also a self-reactive / multi-reactive antibody, and we have prepared 4E10 SCFV-Fc in an experiment for a positive control antibody. The test steps are as follows:
[0100] (1) Package: The ELISA board in the ANA kit has been carried out;
[0101] (2) Anti-anti - 50 μg / mL SCFV-Fc protein 50 μl, room temperature reaction for 2 h;
[0102] (3) Secondary antibody: Abandon the liquid, PBST was washed 3 times, add HRP-labeled sheep anti-human IgG-Fc antibody, and at room temperature 1 h;
[0103] (4) Selective: Abandon the liquid, PBST was washed 3 times, add TMB substrate solution, 100 μL per...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com