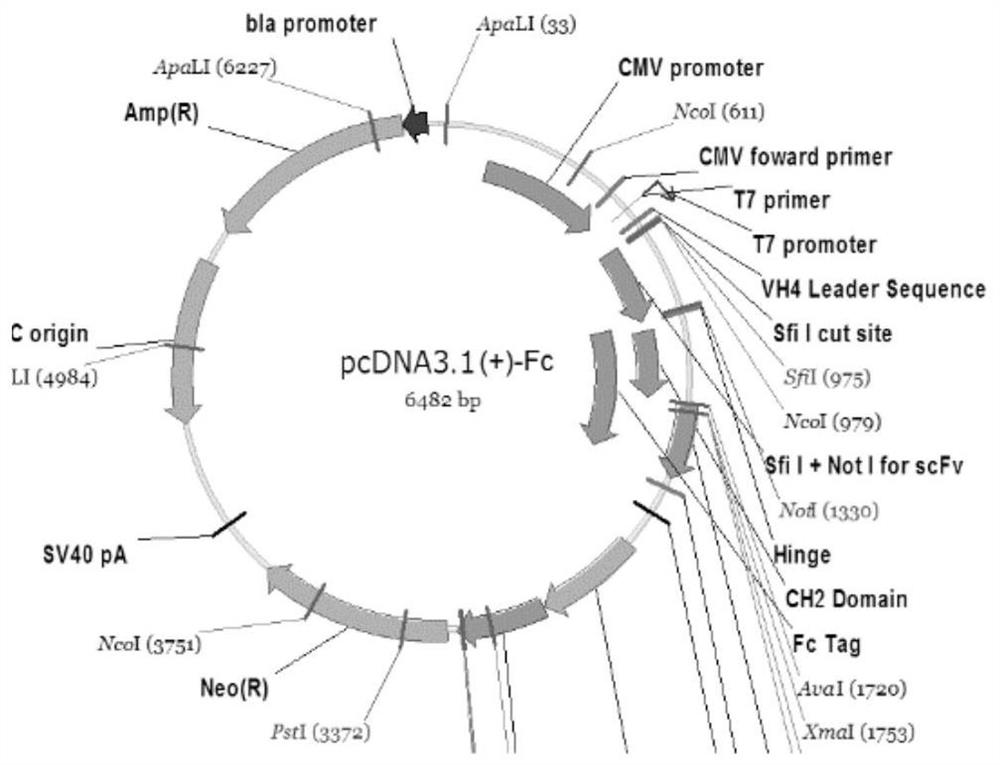
Anti-HIV broad-spectrum neutralizing antibody
An antibody and carrier technology, applied in the fields of cellular immunology and genetic engineering, can solve the problems of poor immunogenicity and virus mutation, and achieve the effect of good application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The preparation of embodiment 1 anti-HIV antibody
[0068] 1. Sorting of mature B cells
[0069] Buffer1: 500ml 1×PBS+2ml 0.5M EDTA+25ml 10%BSA (containing 2mM EDTA, 0.5%BSA)
[0070] (1) Collect 200 μl of peripheral blood from humanized BLT mice and add 200 μl of Buffer1;
[0071] (2) Add 10 times the volume of ACK lysing buffer (Fisher / BioWhittaker), incubate at room temperature for 10 minutes, and centrifuge at 1500 rpm for 10 minutes;
[0072] (3) Discard the supernatant, add 10ml Buffer 1 to wash the cells, and centrifuge at 1500rpm for 10min;
[0073] (4) Discard the supernatant, add 10 μl Buffer 1 to resuspend the cells; add the following flow cytometry antibodies to label mature B cells:
[0074] anti-CD5 / FITC (UCHT2, eBioscience)
[0075] anti-CD19 / PE (SJ25-C1, BD Pharmingen)
[0076] anti-CD10 / APC (BC96, eBioscience)
[0077] anti-CD27 / PE-Cy7 (O323, eBioscience)
[0078] anti-IgM / PE-Cy5 (G20-127, BD Pharmingen)
[0079] (5) Sorting mature B cells (CD5-...
Embodiment 2
[0097] The detection of embodiment 2 antibody characteristics
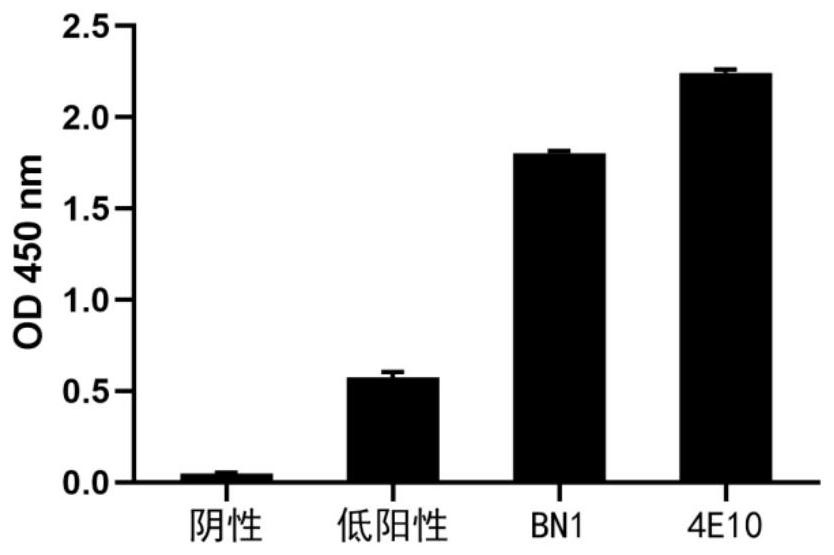
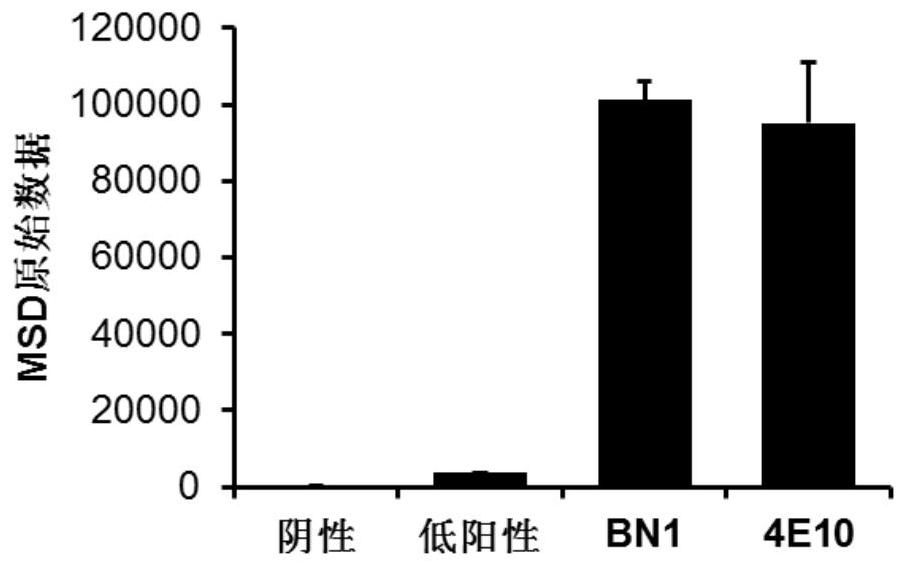
[0098] 1. Self-reactivity detection
[0099] Antibody self-reactivity was detected using a clinical standard antinuclear antibody detection kit (QUANTA Lite TM ANAELISA, INOVA Diagnostics, Inc.) were tested, and negative reaction samples, low positive reaction samples, and high positive reaction samples were provided in the detection kit. 4E10 is an HIV broad-spectrum neutralizing antibody and also an autoreactive / polyreactive antibody. In the experiment, we prepared 4E10 scFv-Fc as a positive control antibody. The detection steps are as follows:
[0100] (1) Coating: the Elisa plate in the ANA kit has been coated with antigen;
[0101] (2) Primary antibody: Add 50 μl of 50 μg / ml scFv-Fc protein, react at room temperature for 2 hours;
[0102] (3) Secondary antibody: Discard the liquid in the well, wash 3 times with PBST, add HRP-labeled goat anti-human IgG-Fc antibody, and react at room temperature for 1 hour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com