Rosuvastatin calcium pharmaceutical preparation
A technology for rosuvastatin calcium and pharmaceutical preparations, applied in the field of rosuvastatin calcium pharmaceutical preparations, can solve the problems of poor stability of rosuvastatin calcium, low dissolution rate of preparations, and poor dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
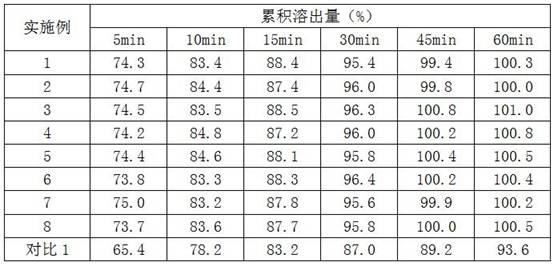
Examples
Embodiment 1
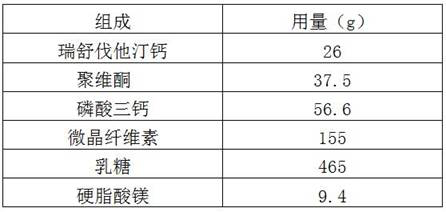
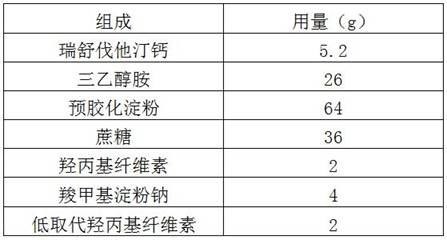
[0032]
[0033] Preparation:
[0034] (1) Mix rosuvastatin calcium, microcrystalline cellulose, lactose, povidone and crospovidone evenly;
[0035] (2) Add triethanolamine to step (1) and mix evenly to obtain a mixture;
[0036] (3) wet granulating the mixture in step (2), drying and sizing to obtain drug granules;
[0037] (4) Compress the drug granules into tablets.
Embodiment 2
[0039]
[0040] Preparation:
[0041] (1) Mix rosuvastatin calcium, microcrystalline cellulose, lactose, povidone and crospovidone evenly;
[0042] (2) Add triethanolamine to step (1) and mix evenly to obtain a mixture;
[0043] (3) wet granulating the mixture in step (2), drying and sizing to obtain drug granules;
[0044] (4) Compress the drug granules into tablets.
Embodiment 3
[0046]
[0047] Preparation:
[0048] (1) Mix rosuvastatin calcium, microcrystalline cellulose, lactose, povidone and crospovidone evenly;
[0049] (2) Add triethanolamine to step (1) and mix evenly to obtain a mixture;
[0050] (3) wet granulating the mixture in step (2), drying and sizing to obtain drug granules;
[0051] (4) Compress the drug granules into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com