Applications of semifluid containing blood cells, vaccine containing semifluid and preparation method of vaccine
A technology of blood cells and semi-fluids, applied in the field of semi-fluids containing blood cells, vaccines containing the semi-fluids and the preparation of the vaccines, to achieve the effects of low immune activity, low graft risk, and high effectiveness against solid tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
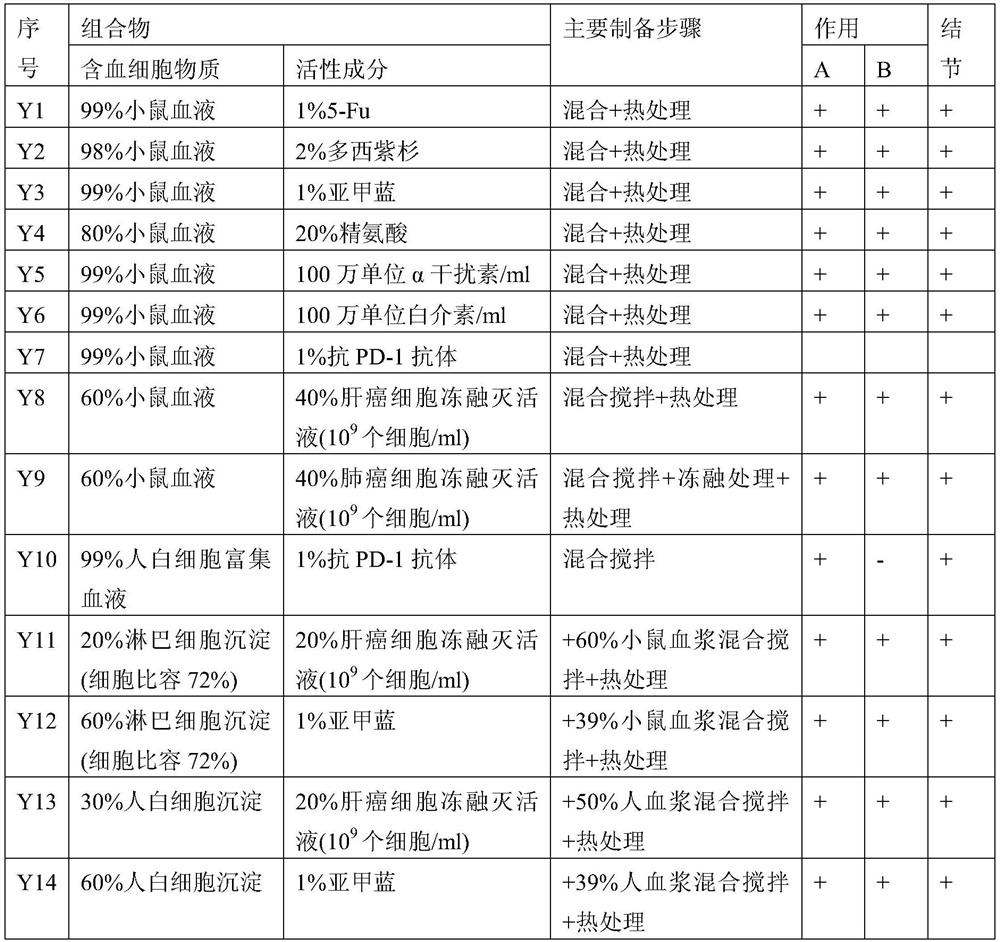
[0121] According to yet another aspect disclosed in the present application, it provides a method for preparing a vaccine for treating or inhibiting solid tumors, the vaccine comprising a semifluid containing blood cells as an antigen, the method comprising the following steps:
[0122] a. providing fluid containing blood cells;
[0123] b. Carry out semi-fluidization treatment to the fluid in step a, wherein said semi-fluidization is selected from one or more of the following: semi-fluid viscosification of liquid, semi-fluid solidification of liquid, non-liquid or solidification broken.
[0124] If involved, the definitions and descriptions of the relevant terms in the foregoing aspects disclosed in the present application are also applicable to this aspect.
[0125] In one embodiment, the step a may include the step of: providing the fluid containing blood cells from natural blood or concentrated blood.
[0126] In one embodiment, the step a may include the following steps...
Embodiment 1
[0163] Embodiment 1: the preparation of vaccine
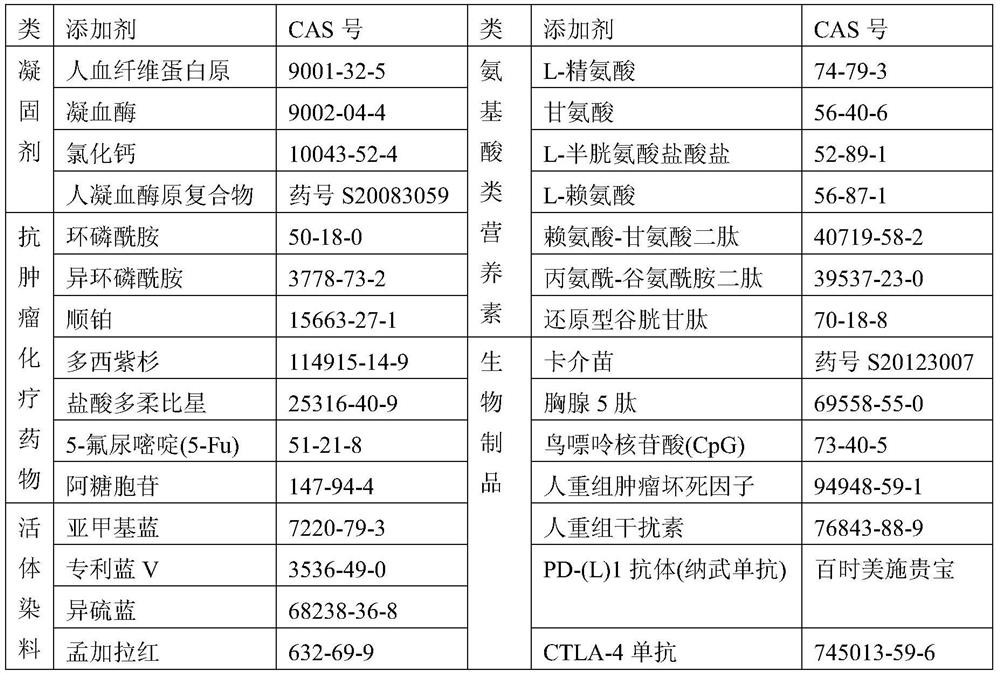
[0164] In the following examples, the blood cells used are selected from one or more of the following groups:
[0165] 1) Blood cells in the blood. For example, using blood containing the desired blood cells;
[0166] 2) Desired blood cells enriched in the blood fraction. For example, using blood cell-rich blood cells to concentrate blood;
[0167] 3) Natural blood cell preparations and / or engineered blood cells derived from organs and / or tissues rich in blood cells. Natural cell preparations include purified natural cells and their derivatives. For example, cell pellets, leukocyte pellets, red blood cell pellets, platelet pellets obtained from natural blood separations according to the prior art. Another example is hematopoietic stem cells extracted and prepared from bone marrow and other tissues according to the prior art. For another example, through in vitro induction, activation, and expansion of autologous or alloge...
Embodiment 1a
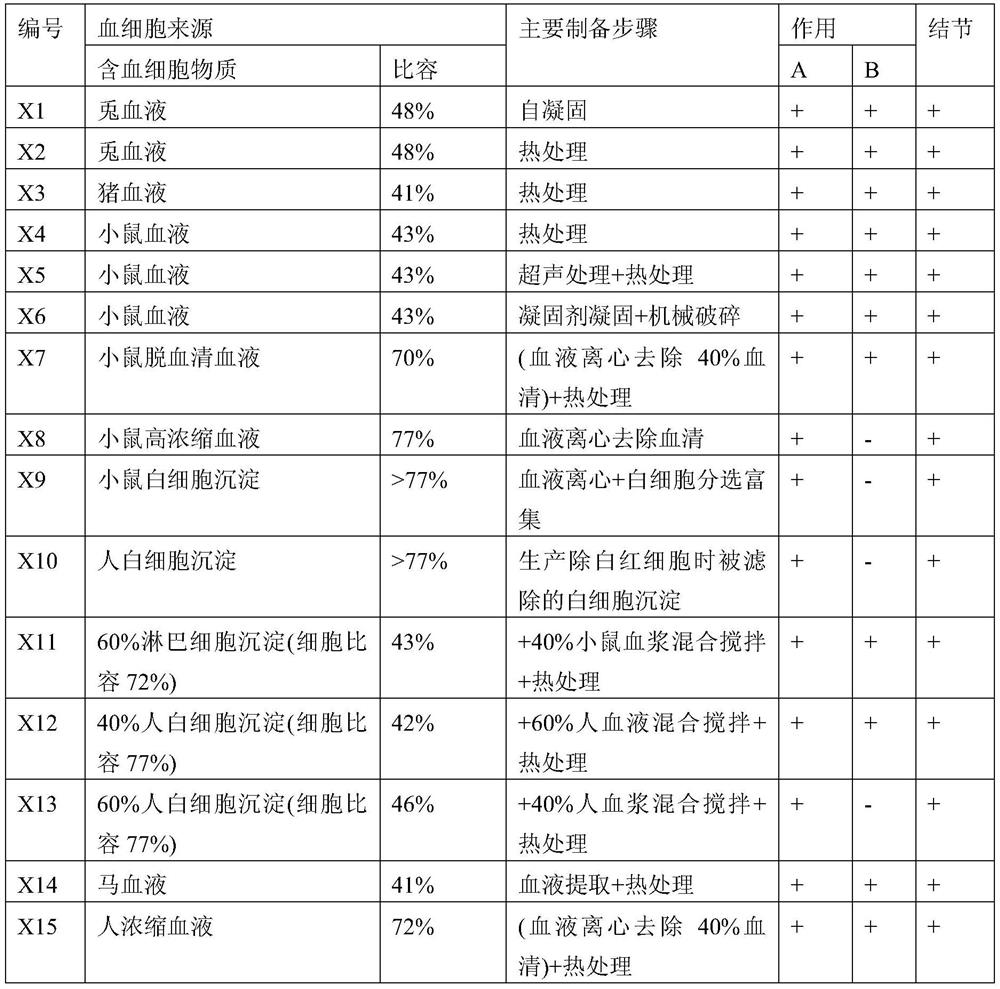
[0177] Example 1a: Kill a New Zealand rabbit and take 15ml of the free blood, and let it stand at room temperature for 30 minutes to obtain self-coagulated blood (X1 in Table 2).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com