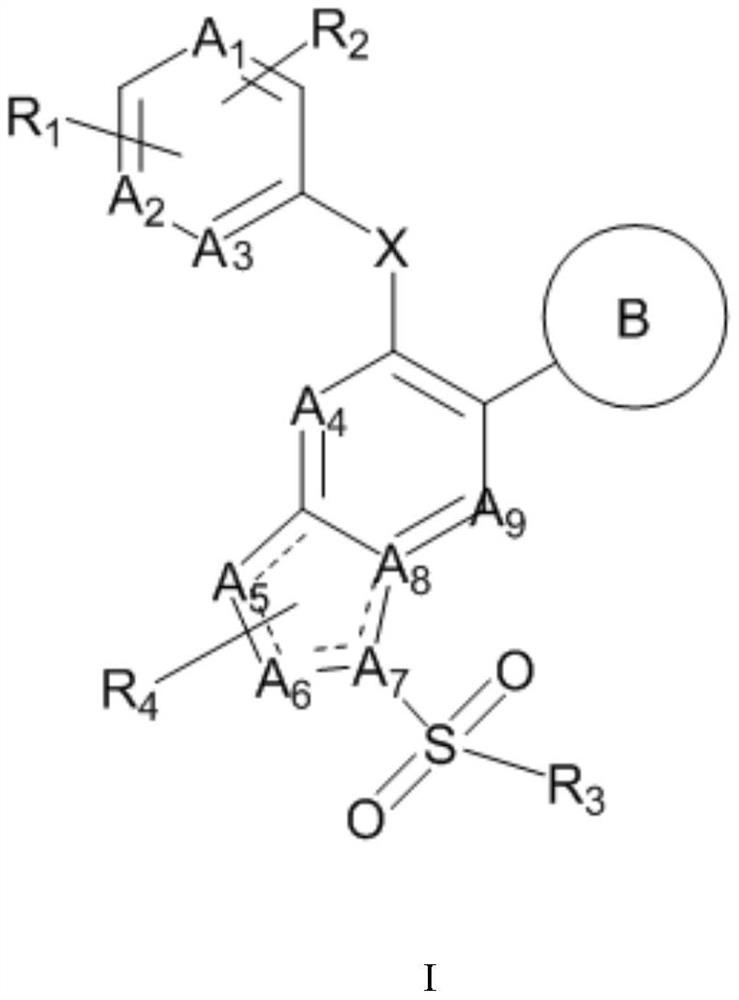
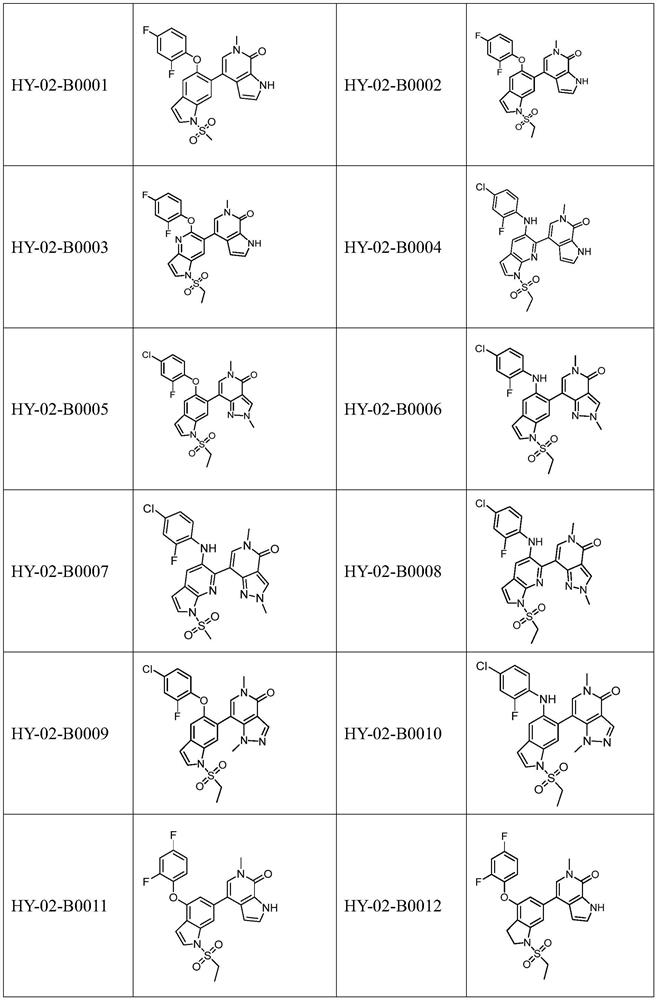
Nitrogen-containing heterocyclic compound as well as preparation method and application thereof
A technology of nitrogen heterocyclic compounds and compounds, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0123] Preparation Example 1: Intermediate 1 (INT-01): 6-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-2- base)-1,6-dihydro-7H-pyrrolo[2,3-c]pyridin-7-one
[0124]
[0125] The first step: 4-bromo-6-methyl-1,6-dihydro-7H-pyrrolo[2,3-c]pyridin-7-one (I-02)
[0126] Compound I-01 (3.0g) and NaOH (1.5g) were dissolved in ethanol / distilled water (20mL / 20mL), stirred overnight at room temperature, LCMS monitoring was completed, the reaction solution was concentrated, extracted with EA, the organic phase was dried and concentrated, The crude product I-02 (1.3 g) was obtained directly. LCMS (ES, m / z): 227.1 [M+H] + .
[0127] The second step: 6-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,6-dihydro- 7H-pyrrolo[2,3-c]pyridin-7-one compound I-02 (1.3g), compound pinacol diboronate (2.7g), Pd 2 (dba) 3 (120mg), X-phos (250mg) and KOAc (1.3g) were dissolved in dioxane (25mL), stirred overnight at 80°C, LCMS monitoring was complete, the reaction solution was extra...
preparation Embodiment 2
[0129] Intermediate Two (INT-02): 2,5-Dimethyl-7-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) -2,5-Dihydro-4H-pyrazolo[4,3-c]pyridin-4-one
[0130]
[0131] The first step: 1H-pyrazolo[4,3-c]pyridin-4-ol (I2-02)
[0132] Compound I2-01 (5.0g) and H 2 O (2.0 mL) was added to ACOH (200 mL), stirred at 100°C overnight, LCMS detected that the reaction was complete, the solvent was spin-dried, the residue was dissolved in methanol, filtered, and the filtrate was concentrated to directly obtain compound I2-02 (4.2 g). LCMS (ES, m / z): 136.0 [M+H] + .
[0133] The second step: 7-bromo-1H-pyrazolo[4,3-c]pyridin-4-ol (I2-03)
[0134] Compound I2-02 (4.2g) was added to ACOH (80mL), stirred at room temperature, and then Br 2 (1.8ml) was added to the reaction, stirred at room temperature overnight, LCMS detected that the reaction was complete, the solvent was directly concentrated and spin-dried to obtain compound I2-03 (2.5g). LCMS (ES, m / z): 214.0 [M+H] + .
[0135] The third...
preparation Embodiment 3
[0140] INT-03: 1,5-Dimethyl-7-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,5- Dihydro-4H-pyrazolo[4,3-c]pyridin-4-one
[0141]
[0142] The first step: 7-bromo-1,5-dimethyl-1,5-dihydro-4H-pyrazolo[4,3-c]pyridin-4-one (I3-01)
[0143] Compound I2-03 (2.5g), CH 3 I (5.0g) and CS 2 CO 3(9.5g) was dissolved in DMF (80mL), stirred overnight at 50°C, and the reaction was detected by LCMS, then the reaction solution was added to EA, washed with distilled water (50mL*3), and the organic phase was washed with anhydrous Na 2 SO 4 After drying, filtration and concentration, the residue was separated and purified by silica gel chromatography (PE:EA=1:3) to obtain compound I3-01 (500 mg). LCMS (ES, m / z): 242.0 [M+H] + .
[0144] The second step: 1,5-dimethyl-7-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,5- Dihydro-4H-pyrazolo[4,3-c]pyridin-4-one (INT-03)
[0145] Compound I3-01 (450mg), pinacol diboronate (700mg), Pd(PPh 3 ) 4 (220mg) and TEA (600mg) were dissolved in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com