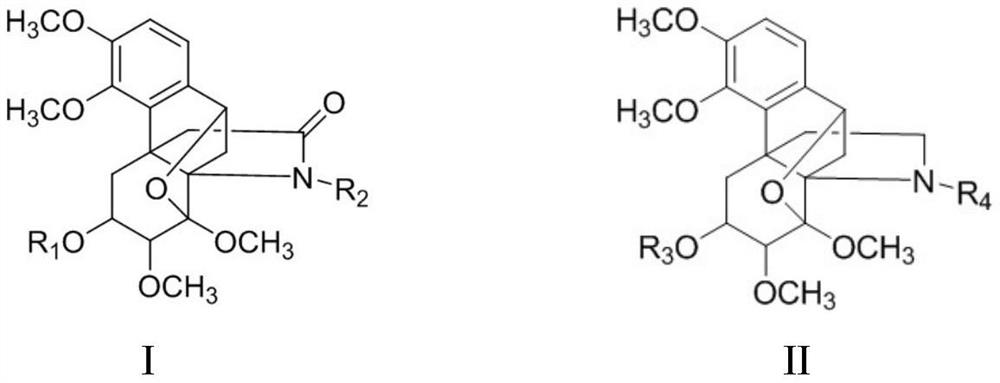
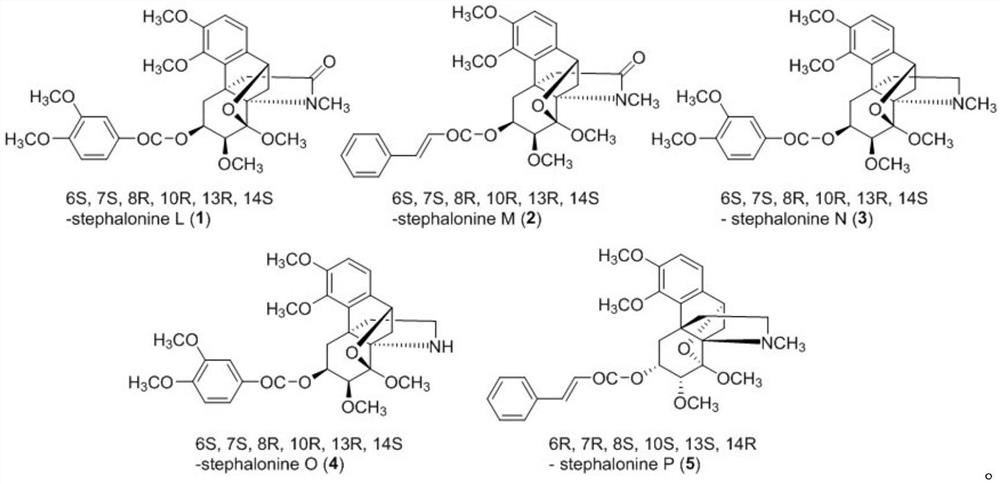
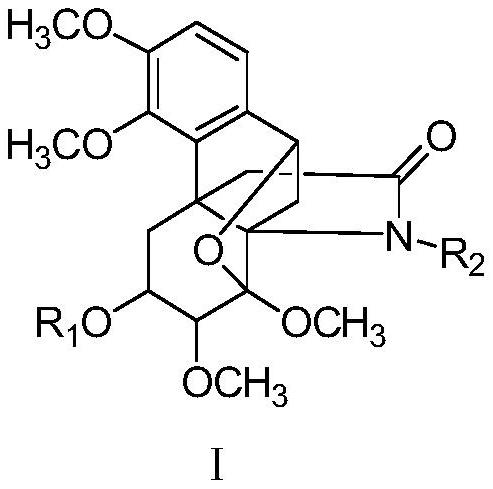
Hasubanane alkaloid compounds as well as preparation method and application thereof
A technology of alkaloids and compounds, applied in the field of medicine, can solve the problems of few reports on structure and biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) 1 kg of Stephania vine was cold-soaked 3 times with 75% ethanol, each time for 24 hours (each dosage: 12 L), and the extract was recovered under reduced pressure to obtain a crude extract;
[0030] (2) The ethanol extract obtained in step (1) is dissolved in water, sherwood oil, methylene chloride, ethyl acetate and n-butanol extraction, the volume ratio of the extract and the extraction solvent is 1:1, and each solvent is extracted 5 times, get the extract;
[0031] (3) The extract obtained in step (2) was separated by silica gel column chromatography, and eluted with a mixed solvent of petroleum ether and ethyl acetate at 100:3, 100:7, 100:10, 100:15, and 100:20;
[0032] (4) The 100:7~100:15 fraction obtained in the above step (3) is separated by ODS chromatography, using methanol / water 1:9, 3:7, 5:5, 7:3, 9:1 as the mobile phase gradient elution;
[0033] (5) The methanol:water (3:7~9:1) fraction obtained in the above step (4) is separated by HPLC-UV chromatog...
Embodiment 2
[0053] (1) 1 kg of Stephania vine was cold-soaked 4 times with 85% methanol, each time for 24 hours (each dosage: 15 L), and the extract was recovered under reduced pressure to obtain a crude extract;
[0054] (2) The ethanol extract obtained in step (1) is dissolved in water, sherwood oil, methylene chloride, ethyl acetate and n-butanol extraction, the volume ratio of the extract and the extraction solvent is 1:1, and each solvent is extracted 4 times, Get extracts of different polar parts;
[0055] (3) The extract obtained in step (2) was separated by silica gel column chromatography, and eluted with a mixed solvent of petroleum ether and ethyl acetate at 100:3, 100:7, 100:10, 100:15, and 100:20;
[0056] (4) The 100:7~100:15 fraction obtained in the above step (3) is separated by ODS chromatography, using methanol / water 1:9, 3:7, 5:5, 7:3, 9:1 as the mobile phase gradient elution;
[0057] (5) The methanol:water (3:7~9:1) fraction obtained in the above step (4) is separat...
Embodiment 3
[0060] (1) 1 kg of Stephania vine was refluxed 4 times with 100% ethanol, each time for 24 hours (each dosage: 12 L), and the extract was recovered under reduced pressure to obtain a crude extract;
[0061] (2) The ethanol extract obtained in step (1) is dissolved in water, sherwood oil, dichloromethane, ethyl acetate and n-butanol extraction, the volume ratio of the extract and the extraction solvent is 1:1, and each solvent is extracted 5 times, Get extracts of different polar parts;
[0062] (3) The extract obtained in step (2) was separated by silica gel column chromatography, and eluted with a mixed solvent of dichloromethane and methanol at 100:0, 100:1, 100:3, 100:5, 100:7, and 100:10;
[0063] (4) The 100:1-100:7 fraction obtained in the above step (3) was separated by ODS chromatography, and the mobile phase was gradient eluted with acetonitrile / water 1:9, 3:7, 6:4, and 8:2;
[0064] (5) Acetonitrile obtained in the above step (4): water (3:7~8:2) fraction is separat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com