FX ACTIVATION PROCESS AND USE THEREOF IN PREPARATION OF FXa COMPOSITION
An activation method and composition technology, applied in chemical instruments and methods, biochemical equipment and methods, peptide preparation methods, etc., can solve problems such as prolonged blood coagulation time and increased blood loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
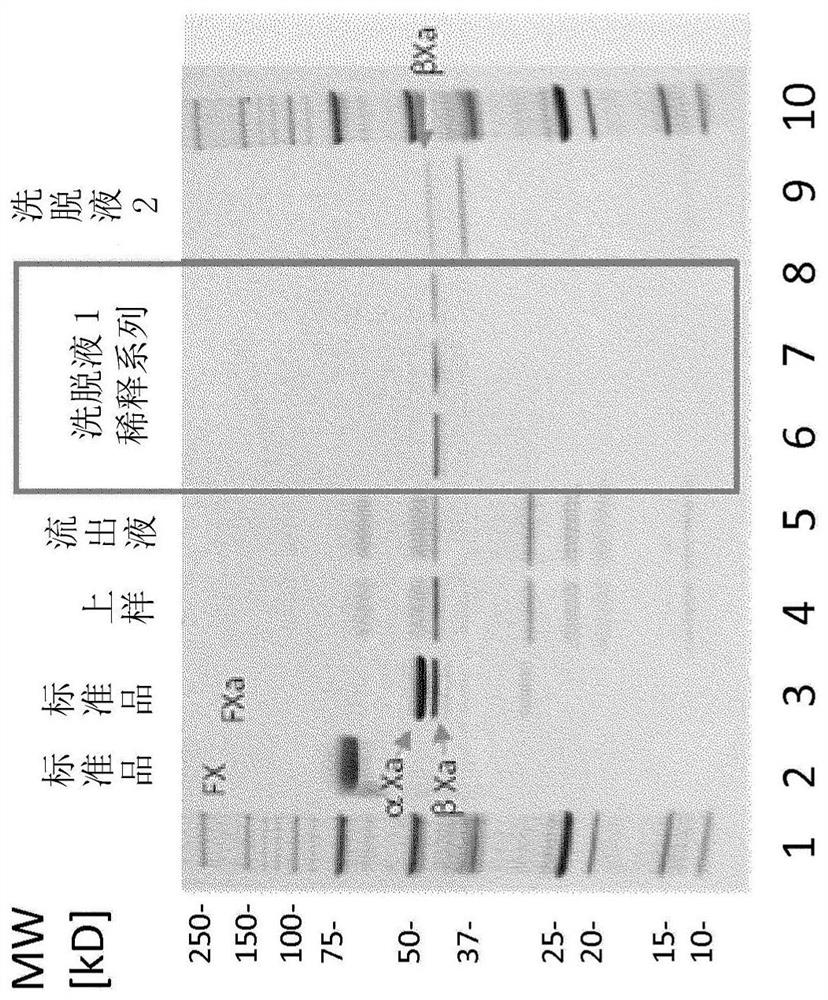
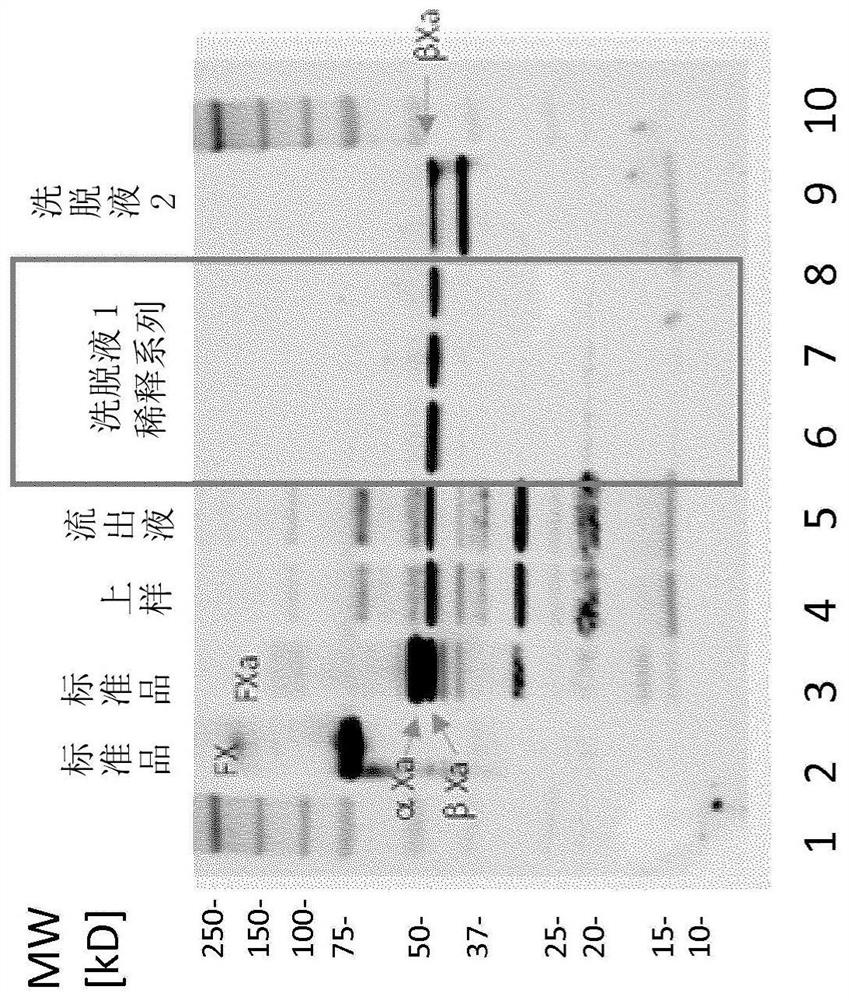
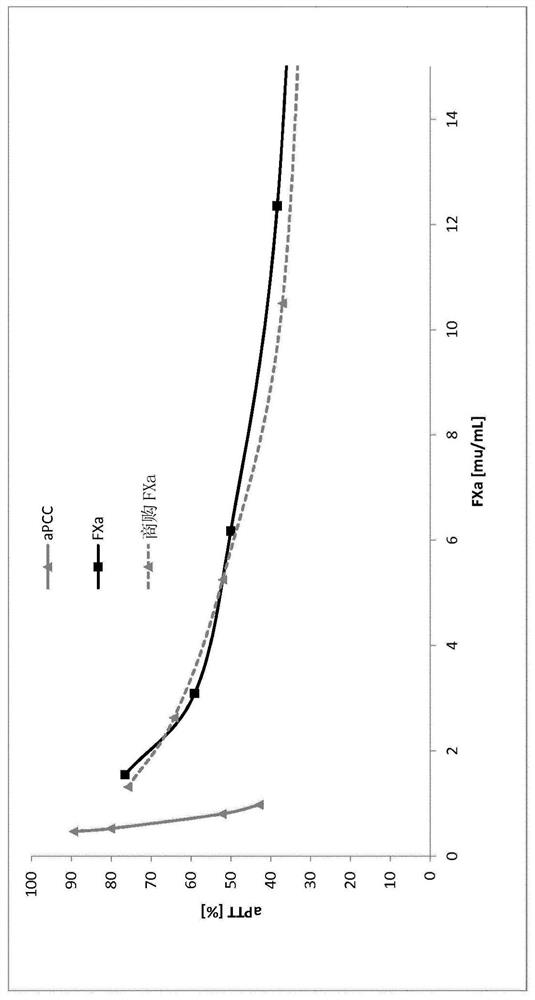
Image
Examples
example 1
[0147] Example 1 (Activation)
[0148] Mix 40 mg of synthetic phosphatidylserine (SPS) with 10 ml of reaction buffer (20 mM TRIS and 150 mM NaCl at pH = 7.5), and use Disperse for 30 minutes and cool to room temperature to obtain SPS dispersion.
[0149] 55ml of the initial protein mixture containing 2mg / ml total protein, about 40IU / ml FX and about 0.5U / ml FII was mixed with 27.5ml of SPS dispersion, 27.5ml of reaction buffer and 55ml of reaction buffer containing 75mM calcium ions. The pH 7.5 mixture was incubated at room temperature for 6 hours with gentle agitation before adding 55 ml of reaction buffer with 200 mM EDTA. The dispersed PS was dissolved by adding 1.5 mM Triton X-100, and the resulting clear solution was filtered through a 0.45 μm PES membrane filter to recover the FXa-containing intermediate.
[0150]The mixture thus prepared, as defined in the assay instructions, had a FXa-specific activity of about 9.6 IU / ml, a thrombin level of less than 50 IU / ml, and...
example 2
[0151] Example 2 (Activation)
[0152] 100 ml of the initial protein mixture containing 40 mg / ml total protein, about 60 IU / ml FX and about 60 U / ml FII was mixed with 90 ml of reaction buffer and 100 ml of reaction buffer containing 75 mM Ca ions. 10 ml of the PS dispersion were added dropwise in a continuous operation. The pH 7.0 mixture was incubated at 22° C. for 16 hours with gentle agitation before adding 100 ml of reaction buffer with 200 mM EDTA. The dispersed PS was dissolved by adding 1.5 mM Triton X-100, and the resulting clear solution was filtered through a 0.45 μm PES membrane filter to recover FXa-containing intermediates.
[0153] The FXa-specific activity of the mixture thus prepared was 8.2 IU / ml, the FIIa-specific activity was 619 IU / ml, and the total protein content was 9.9 mg / ml. The yield of FXa was 33% based on the FX content activated by PS and Ca ions.
example 3
[0154] Example 3 (Activation)
[0155] The initial protein mixture was diluted with reaction buffer, mixed with PS dispersion and Ca ions to obtain a solution containing 40 IU / ml FX, 0.33 mg / ml PS and 25 mM Ca ions. The mixture was incubated at 15°C for 10 hours with gentle agitation, then the reaction was terminated with 50 mM EDTA. The dispersed PS was dissolved by adding 1.5 mM Triton X-100, and the resulting clear solution was filtered through a 0.45 μm PES membrane filter to recover FXa-containing intermediates.
[0156] The FXa-specific activity of the mixture thus prepared was 4.1IU / ml, the FIIa-specific activity was 3.1IU / ml, and the total protein content was 0.4mg / ml. Based on the FX content activated by PS and calcium ions, the FXa yield was 41 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com