Tumor enzyme response type recombinant pyroptosis protein drug delivery system and anti-tumor purpose thereof
A protein and fusion protein technology, applied in the field of biomedicine, can solve problems such as difficulty in ensuring the efficiency of pyroptosis into cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
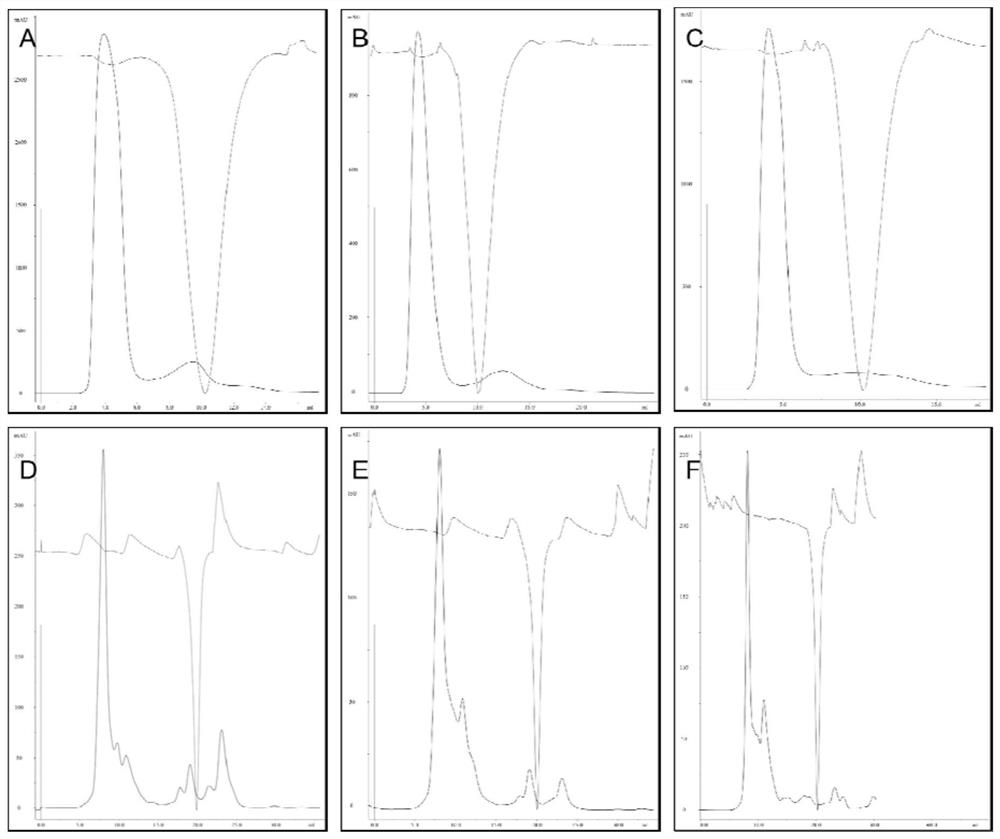
[0181] Example 1: Purification results of FPLC
[0182] Primin (GSDMA3, GSDMA3-PTN and GSDMA3-TAT-PTN), which was preliminaryly purified by nickel column, and Hitrap Desalting ( figure 1 A-C) and gel exclusion layers (Superdex 75) (SUPERDEX 75) figure 1 D-f) was purified. The peak shape and peak position of three proteins are basically consistent, which is preliminarily indicated that the protein transformed by genetic engineering has not affected its structure and function.
Embodiment 2
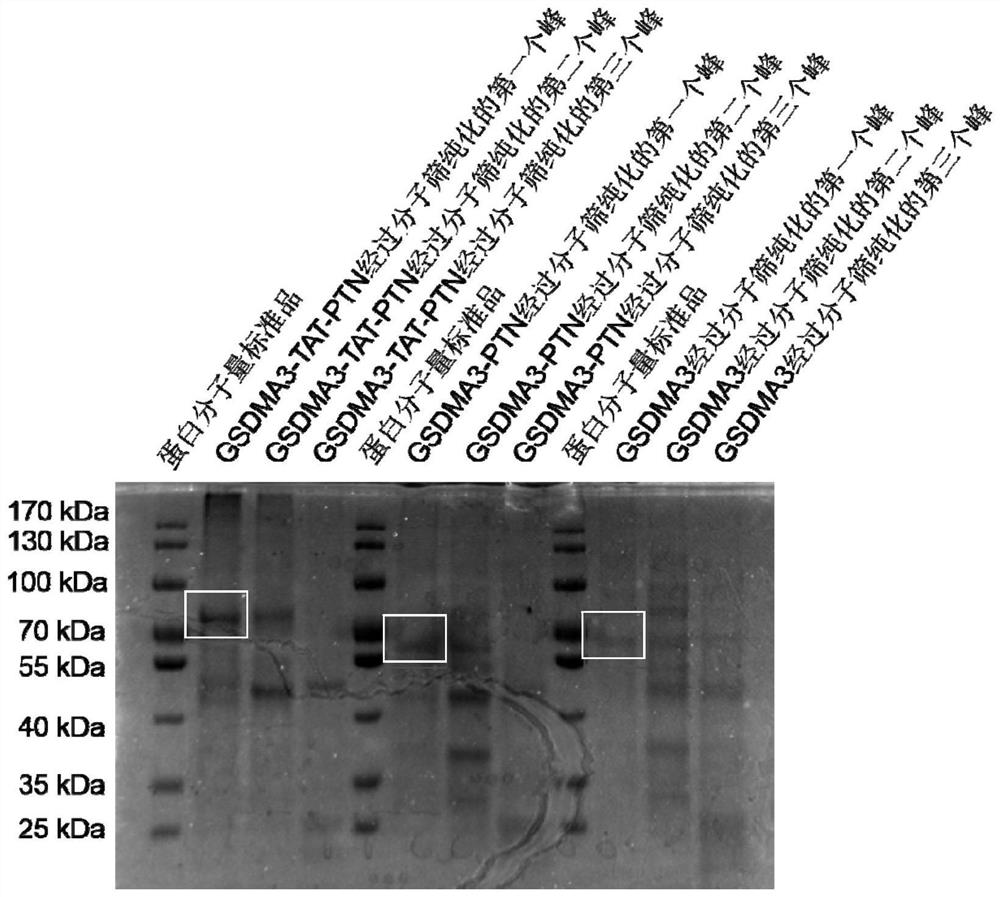
[0183] Example 2: Purification and identification of fusion proteins
[0184] Samples of the top three absorption peaks of Superdex 75 were verified by SDS-PAGE electrophoresis. figure 2 Indicated. Lanes 1-9 are proteins of three absorption peaks of GSDMA3-TAT-PTN, GSDMA3-PTN, and GSDMA3, respectively. The strips of GSDMA3 are in line with a theoretical molecular weight of approximately 55kda (lane 7); GSDMA3-PTN strip (lane 4) Basic and GSDMA3 strips are basically at the same level, indicating that the molecular weight of both is close and theoretical; recombinant protein GSDMA3 -Tat-PTN strip (lane 1) has a significant movement; while other lanes have a conveyor, which indicates that the protein purity of the first absorption peak is better, while the other two absorption peaks have a large number of miscellaneous proteins. So subsequent experiments are used by the first absorption peak.
Embodiment 3
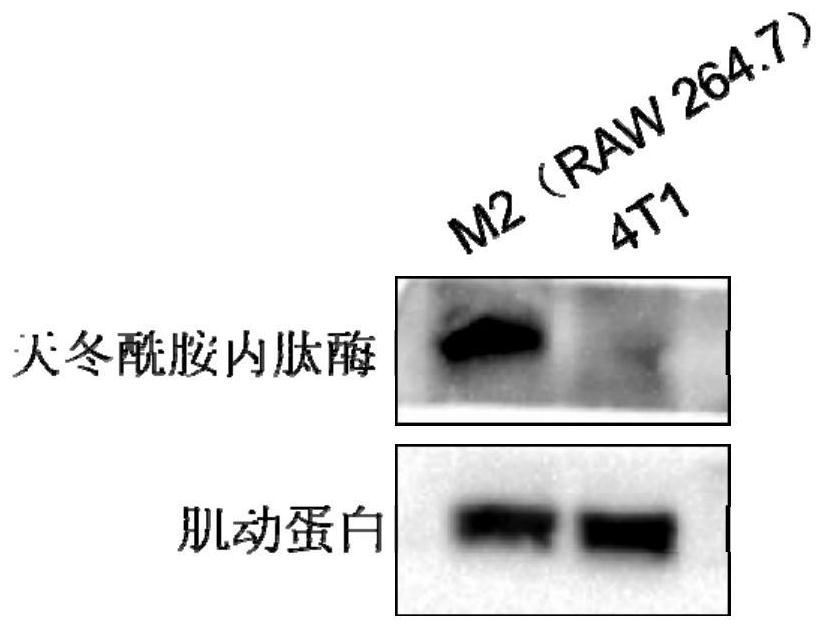
[0185] Example 3: WB detection of cell LeGumain expression level
[0186]The Legumain expression levels of RAW 264.7 and 4T1 cells induced into M2 macrophages were detected using Western blot. Such as image 3 As shown, LeGumain is highly expressed in tumor-related macrophages, which in vitro cultured 4T1 cells in vitro showed low expression of LeGumain enzyme.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com