Chimeric antigen receptor targeting CD22 and CD19 and application thereof
A chimeric antigen receptor, CD22 technology, applied in the field of biomedicine, can solve problems such as poor killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
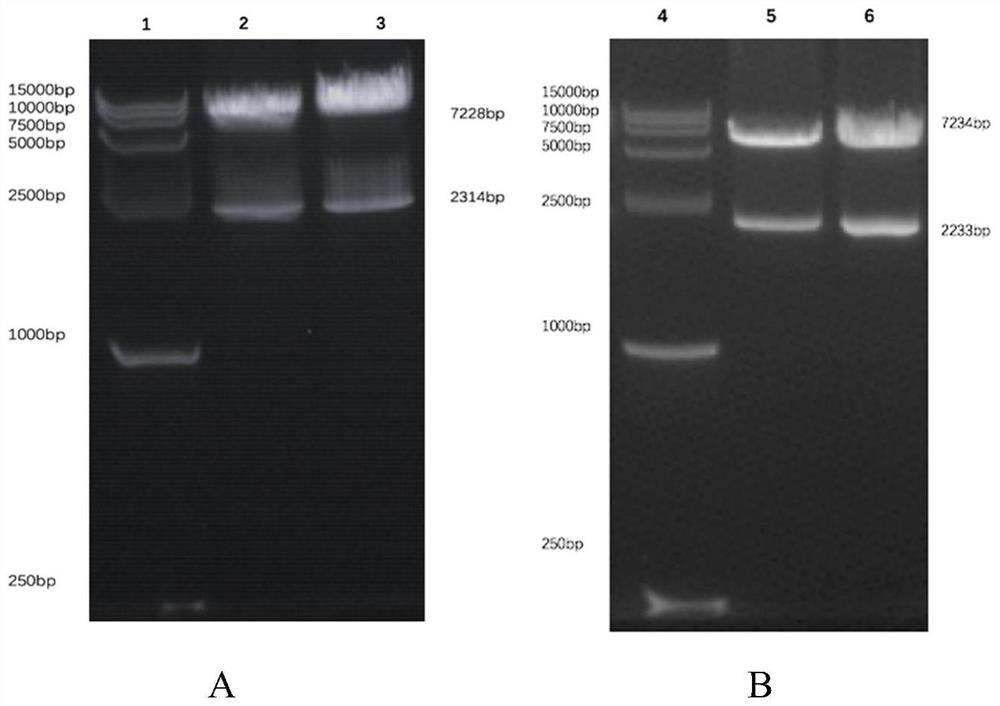
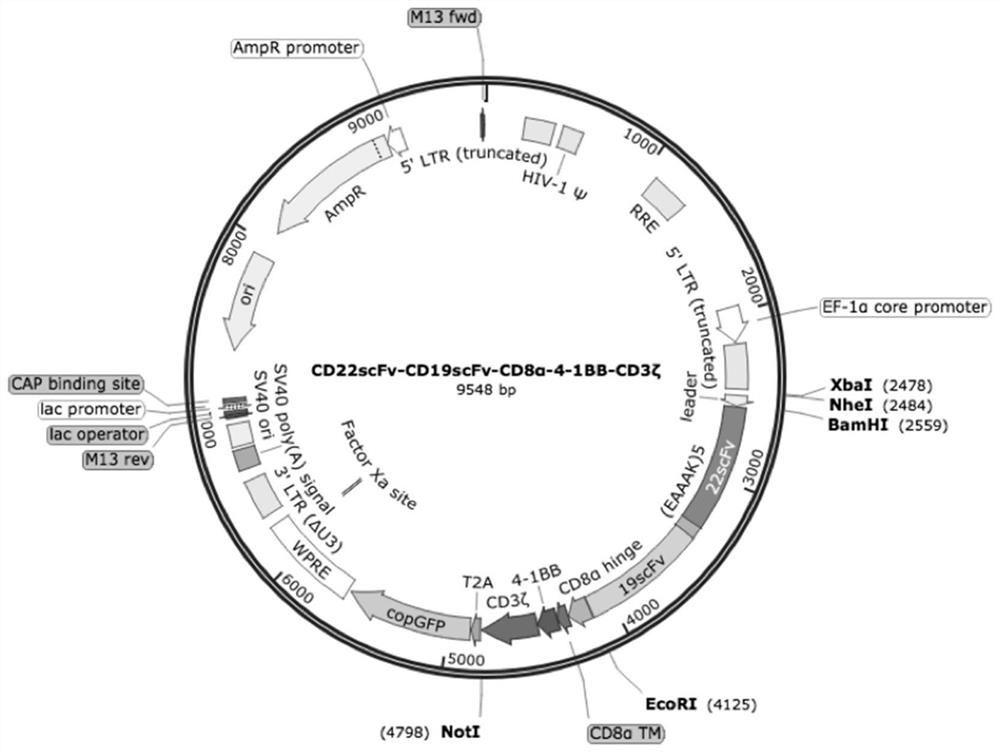
[0086] Example 1: Construction of dual-target chimeric antigen receptor vector
[0087] 1. Using BamH I and EcoR I endonucleases to digest the plasmid containing the CD8α-4-1BB-CD3ζ fragment constructed by the inventor in the early stage to obtain the CD8α-4-1BB-CD3ζ fragment, the amino acid sequence of which is as shown in SEQ ID NO.5 shown. The plasmid containing the CD8α-4-1BB-CD3ζ fragment can be prepared by any suitable method in the prior art, for example, the patent No. ZL201510233748.0.
[0088] 2. The synthesized CD 22scFv-CD 19scFv fragment, CD 19scFv-CD22scFv, CD19V L -CD22V L -CD22V H -CD19V H and CD22V L -CD19V L -CD19V H -CD22V H The fragments were respectively connected to the target vector, and the constructed CD22scFv-CD19scFv-CD8α-4-1BB-CD3ζCAR (22-19CAR), CD19scFv-CD22scFv-CD8α-4-1BB-CD3ζCAR (19-22CAR), CD19V L -CD22V L -CD22V H -CD19V H -CD8α-4-1BB-CD3ζ(19╳22CAR-T) and CD22V L -CD19V L -CD19V H -CD22V H -CD8α-4-1BB-CD3ζ(22╳19CAR-T) destinati...
Embodiment 2
[0089] Example 2: Preparation of dual-target chimeric antigen receptor lentiviral modified T cells
[0090] 1. Use EndoFree Plasmid Maxi Plasmid Extraction Kit (QIAGEN Company) to extract 22-19CAR, 19-22-CAR, 19╳22CAR and 22╳19CAR expression plasmids and packaging plasmids PRSV-Rev, pMDlg-PRRE, pMD.2G respectively . Each CAR plasmid and packaging plasmid (four plasmids) were transfected with PEI transfection reagent (polyscience company) at a ratio of 12.2:4.11:8.75:3.5 (see the instruction manual of PEI transfection reagent for specific methods). Replace the fresh culture medium 12 hours after transfection, collect the virus supernatant 24 hours and 48 hours later, centrifuge at 4°C, 3000rpm for 15 minutes, filter through a 0.45μm filter, and use 50000g, 4°C, 1.5 hours after ultracentrifugation Concentrate 10 times, then transfer to -80°C for storage.
[0091] 2. Preparation of T cells: Take 10 ml of fresh healthy human peripheral blood, and use RosetteSep T cell enrichment...
experiment example 1
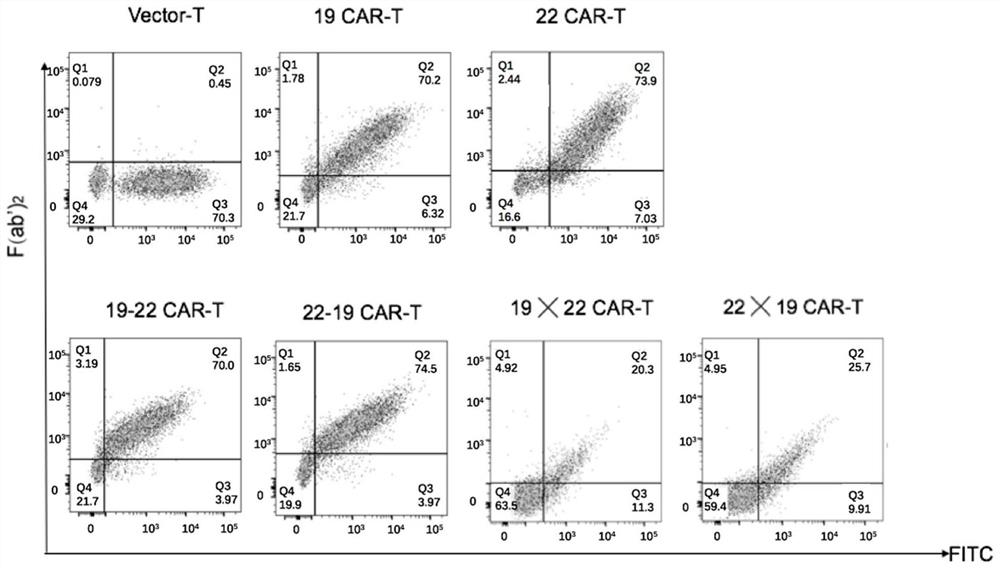
[0094] Experimental example 1: Chimeric antigen receptor 22-19CAR, 19-22-CAR, 19╳22CAR and 22╳19CAR lentiviral modification of T cells to kill leukemia cells
[0095] 1. CD22 expression level in hematological tumor cell lines:
[0096] Both Namalwa and MV4-11 cell lines were purchased from ATCC in the United States. MV4-11-CD19 and MV4-11-CD22 are monoclonal cell lines screened after MV4-11 cell lines were infected with CD19 and CD22, respectively. After cultured separately, draw 5×10 5 The cell suspension was washed twice with PBS, labeled with PE anti-human CD19 monoclonal antibody and APC anti-human CD22 monoclonal antibody (Biolegend Company), and labeled with PE-isotype and APC-isotype as the control group, and incubated on ice for 30 minutes. The expression levels of CD19 and CD22 in various cell lines were detected by flow cytometry, and the results were as follows: Figure 4 shown. Among them, A is the positive expression rate of CD19 target antigen molecules; B is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com