Method for increasing synthetic reaction rate of ixabepilone
A synthesis reaction, ixabepilone technology, applied in the direction of organic chemistry, can solve the problems of unfavorable product quality and industrial production, slow reaction, incomplete epothilone B reaction, etc., to improve process efficiency and product quality, The effect of speeding up the reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
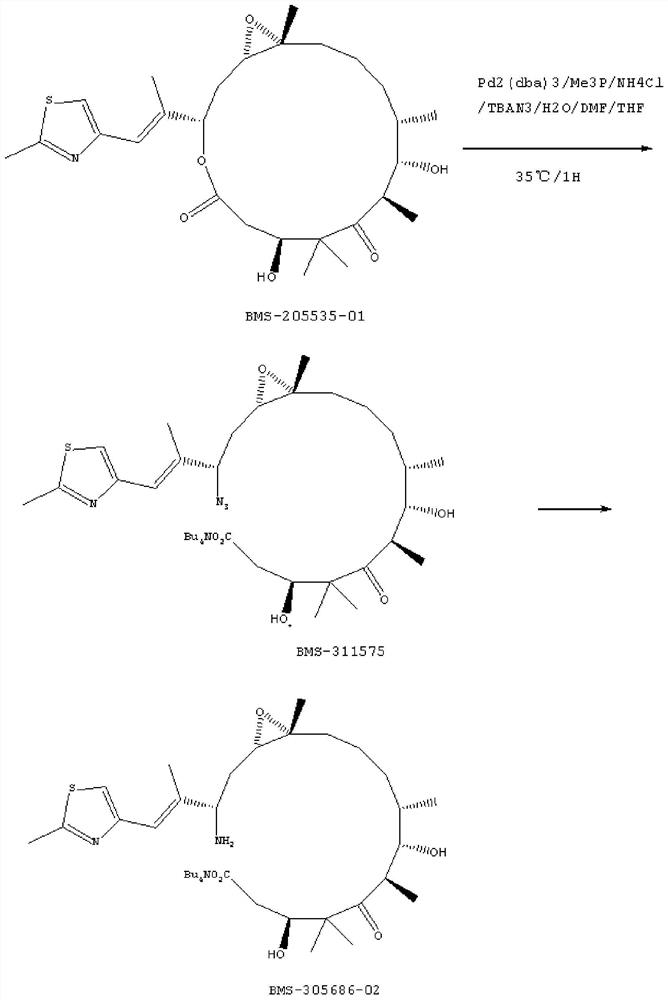
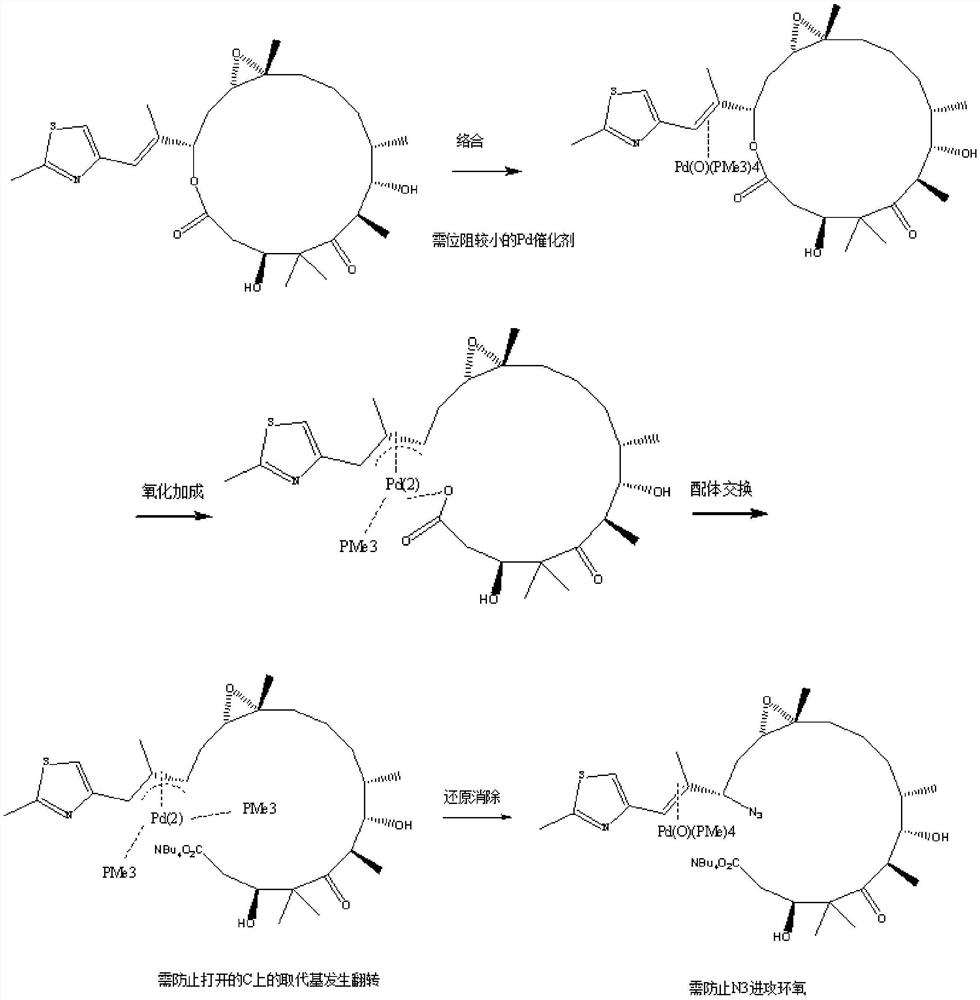
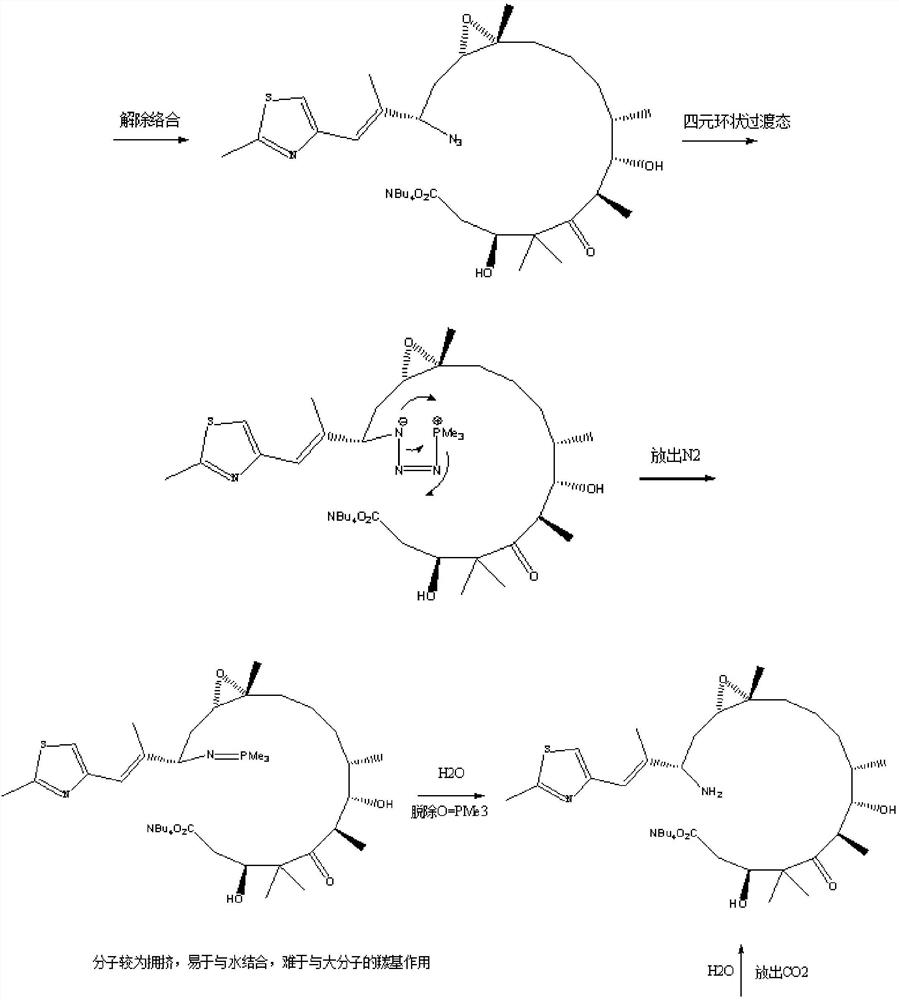
[0029] (1) In 50ml one-mouth bottle A: put 0.5g of epothilone B (1.0eq) into TBAN 3 [i.e. tetrabutylammonium azide] (1.4eq) in THF [i.e. tetrahydrofuran] solution or DMF [i.e. dimethylformamide] solution or aqueous solution (which contains 0.4gTBAN 3 ), shake well to dissolve.
[0030] (2) In the 50ml three-neck bottle B: put 0.08g of Pd 2 (dba) 3 [i.e. tris(dibenzylideneacetone) dipalladium (0)] (0.09eq) and 0.05g of NH 4 Cl [i.e. ammonium chloride] (1eq), protected by argon, heated to 20°C, and then injected 2ml of PMe 3 [Trimethylphosphine] in THF (2.0eq). After stirring for 20 minutes, the solution in bottle A was injected.
[0031] (3) After keeping at 20° C. for 30 minutes, TLC monitors: the raw material point disappears.
[0032] (4) Filtration, and the filter residue was washed once with 3.0 ml of THF to obtain a dark blue clear solution, which was stored under refrigerated conditions at 0-10°C.
[0033] (5) In 50ml one-mouth bottle C: put 1.8ml of DMF and 1.8ml...
Embodiment 2
[0038] (1) In 50ml one-mouth bottle A: put 0.1g of epothilone B (1.0eq) into TBAN 3 (1.4eq) solution (which contains 0.08g TBAN 3 ), shake well to dissolve.
[0039] (2) In the 50ml three-neck bottle B: put 0.02g of Pd 2 (dba) 3 (0.09eq) and 0.015g of NH 4 Cl (1.5eq), protected by argon, heated up to 35°C, and then injected 0.5ml of PMe 3 solution in THF (2.0 eq). After stirring for 20 minutes, the solution in bottle A was injected.
[0040] (3) After keeping at 22° C. for 40 minutes, TLC monitors: the starting point disappears.
[0041] (4) Filtration, and the filter residue was washed once with 1.0 ml of THF to obtain a dark blue clear solution, which was stored under refrigerated conditions at 0-10°C.
[0042] (5) 50ml one-mouth bottle C: put 0.5ml of DMF and 0.5ml of THF in sequence, stir in a water bath at 10°C, then add 0.08g of EDCI (2.2eq) and 0.02g of K 2 CO 3 (0.4eq) and 0.05g of HOBT (1.7eq), stirred for 0.5 hours.
[0043] (6) Add the above-mentioned filt...
Embodiment 3
[0047] (1) In 250ml one-mouth bottle A: put 5g of epothilone B (1.0eq) into TBAN 3 (1.4eq) solution (which contains 4g TBAN 3 ), shake well to dissolve.
[0048] (2) In 250ml three-neck bottle B: put 0.8g of Pd 2 (dba)3 (0.09eq) and 0.25g of NH 4 Cl (0.5eq), protected by argon, heated up to 20°C, and then injected 20ml of PMe 3 solution in THF (2.0 eq). After stirring for 15 minutes, the solution in bottle A was injected.
[0049] (3) After keeping at 20° C. for 50 minutes, TLC monitors: the raw material point disappears.
[0050] (4) Filtration, and the filter residue was washed once with 30 ml of THF to obtain a dark blue clear solution, which was stored under refrigerated conditions at 0-10°C.
[0051] (5) In 500ml one-mouth bottle C: put 20ml of DMF and 20ml of THF in sequence, stir in a water bath at 10°C, then add 4g of EDCI (2.2eq) and 0.7g of K 2 CO 3 (0.4eq) and 2.3g of HOBT (1.7eq), stirred for 0.5 hours.
[0052] (6) Add the above-mentioned filtrate stored ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com