Kit for predicting postoperative survival time of lung squamous carcinoma patient and application of kit
A survival time, lung squamous cell carcinoma technology, applied in the field of genetic engineering, can solve problems such as not giving high reliability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] The invention provides a kit for predicting postoperative survival time of lung squamous cell carcinoma patients. The method includes predicting the survival time of the test sample based on the acquired mutation information of the target region in the genome of the test sample.
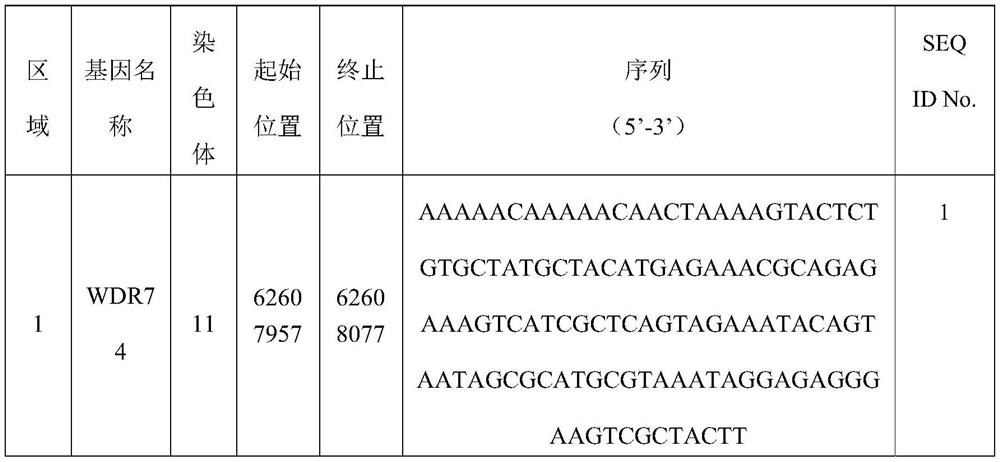
[0121] Wherein, the target area includes a combination of areas 1-14:
[0122] Region 1 is selected from part or all of the 62607957-62608077th base of chromosome 11;
[0123] Region 2 is selected from part or all of the 62608049-62608169 bases of chromosome 11;
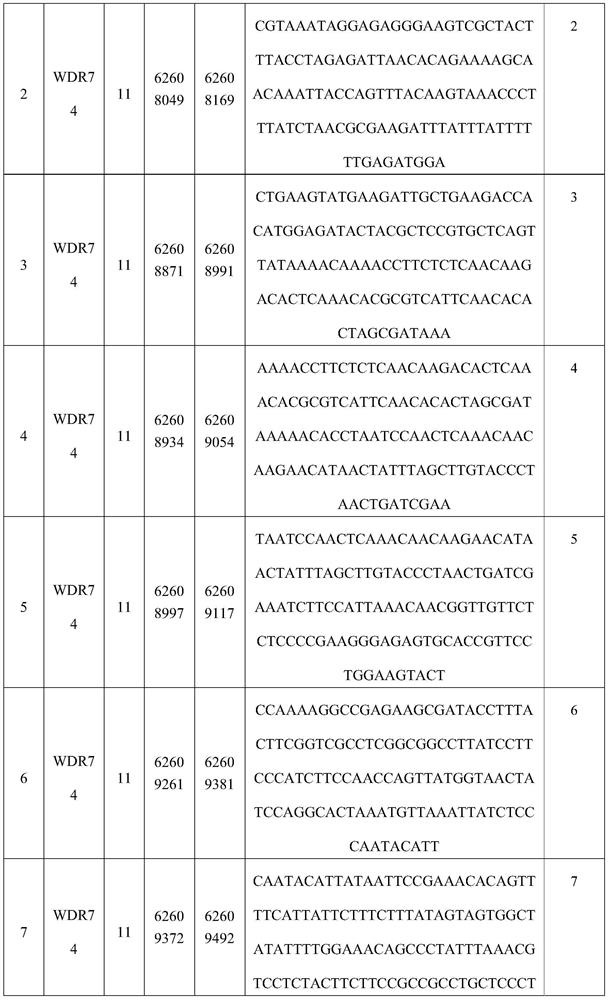
[0124] Region 3 is selected from part or all of the 62608871-62608991 base of chromosome 11;
[0125] Region 4 is selected from part or all of the 62608934-62609054 base of chromosome 11;
[0126] Region 5 is selected from part or all of the 62608997-62609117th base of chromosome 11;
[0127] Region 6 is selected from part or all of the 62609261-62609381 base of chromosome 11;
[0128] Region 7 is selected from part or all of the...
Embodiment 2
[0138] The present invention provides a method for predicting postoperative survival time of lung squamous cell carcinoma patients, which comprises using the kit provided in Example 1 to detect the target area of the sample to be tested.
[0139] The detection method for the target region (the specific non-coding region of the WDR74 gene) can be any general high-throughput sequencing method, such as amplicon sequencing (PCR-based enriched sequencing), or according to the kit provided in Example 1 The probe sequences were subjected to region hybridization sequencing. The length of the region with a coverage greater than 30X in the target region must account for 100% of the total length of the region, and the sequencing results must pass the quality control standards of the sequencing service provider.
[0140] The following are the methods and steps after the sequencing results of the target region are obtained after sequencing:
[0141] a, Using Fastqc to detect the quality...
Embodiment 3
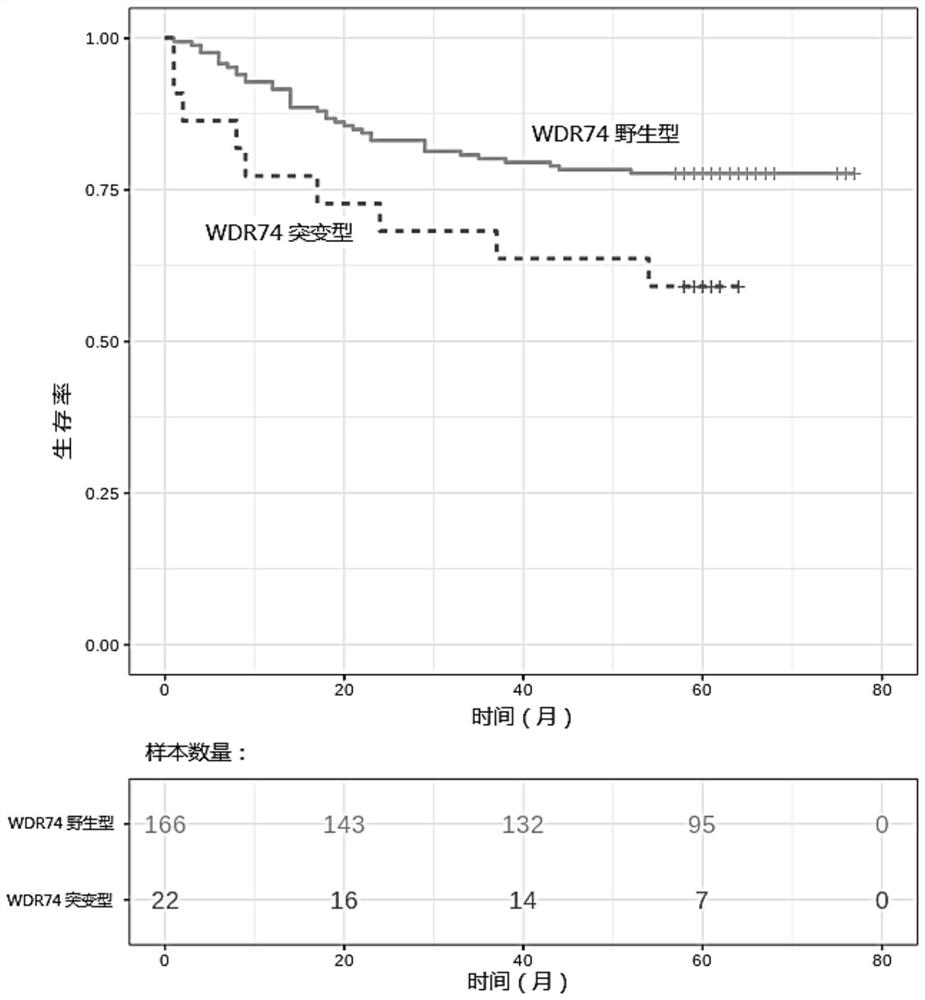
[0149] In this example, 188 lung squamous cell carcinoma patients aged 41-86 were collected, including 71 patients at stage I, 65 at stage II, 50 at stage III, and 2 at stage IV, and determined according to the method provided in Example 2. Whether the patient's tumor tissue carries a mutation in the non-coding region of the WDR74 gene (the target region in Example 1), see Table 2 for detailed clinical information and sequencing results.
[0150] Using Cox univariate analysis, the HR (Hazard ratio, the same below) of the mutation in the non-coding region of the WDR74 gene was 2.13, and the P value was 0.042, which was an unfavorable prognostic factor.
[0151] Using Cox multivariate analysis, combined with gender, age, smoking, cancer stage and other clinical factors, the HR of the mutation in the non-coding region of the WDR74 gene was 2.41, and the P value was 0.02, which was an unfavorable prognostic factor.
[0152] Table 2 Sample clinical information and sequencing result...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com