Human interferon-kappa mutant and preparation method thereof
A mutant and interferon technology, applied in the field of human interferon-κ mutants and its preparation, can solve the problems of free cysteine correct folding and pairing interference, inactivation, etc., to achieve increased binding capacity and protein activity Effect of improving and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1hI
[0037] Embodiment 1 hIFN-κ mutant coding gene and expression vector acquisition
[0038] The amino acid sequence of hIFN-κ is shown below:
[0039] MLDCNLLNVHLRRVTWQNLRHLSSMSNSFPVECLRENIAFELPQEFLQYTQPMKRDIKKAFYEMSLQAFNIFSQHTFKYWKERHLKQIQIGLDQQAEYLNQCLEEDKNENEDMKEMKENEMKPSEARVPQLSSLELRRRYFHRIDNFLKEKKYSDCAWEIVRVEIRR C LYYFYKFTALFRRK (SEQ ID NO: 1)
[0040] According to the hIFN-κ amino acid sequence and its high-level structure published in the prior art, it can be known that the cysteine (Cys) at position 166 does not participate in the formation of disulfide bonds, so the inventors conducted site-directed mutations and constructed mutations The library is to mutate it into any of the other nineteen amino acids that do not contain a sulfhydryl group except Cys among the 20 common amino acids. The mutated sequence is as follows:
[0041] MLDCNLLNVHLRRVTWQNLRHLSSMSNSFPVECLRENIAFELPQEFLQYTQPMKRDIKKAFYEMSLQAFNIFSQHTFKYWKERHLKQIQIGLDQQAEYLNQCLEEDKNENEDMKEMKENEMKPSEARVPQLSSLELRRRYF...
Embodiment 2
[0048] Embodiment 2 protein preparation
[0049] 1. Transfer the recombinant plasmid prepared in Example 1 into Escherichia coli BL21 (DE3) competent cells, screen for kanamycin resistance, pick a single colony and carry out PCR identification, and the positive clone cells selected are those containing the recombinant plasmid pET28 -Engineering bacteria of hIFN–κ-MUT.
[0050] 2. Expression and purification of recombinant plasmid pET28-hIFN–κ-MUT in Escherichia coli BL21(DE3):
[0051] 1) Inoculate a single colony of Escherichia coli BL21(DE3) containing the recombinant plasmid pET28-hIFN-κ-MUT in 5ml of LB liquid medium containing 50μg / ml kanamycin, culture at 37°C with shaking at 200r / min for 16h, Add 5ml of bacterial liquid to 500ml of LB liquid medium containing 50μg / ml kanamycin, and expand the culture at 1:100.
[0052] 2) When the OD600 value of the Escherichia coli cultured in step 1) reaches between 0.6-0.8, add 1 mMIPTG to the bacterial solution, and culture with s...
Embodiment 3
[0056] Example 3 Activity Determination
[0057] 1. Affinity determination of interferon-κ protein binding to its receptor
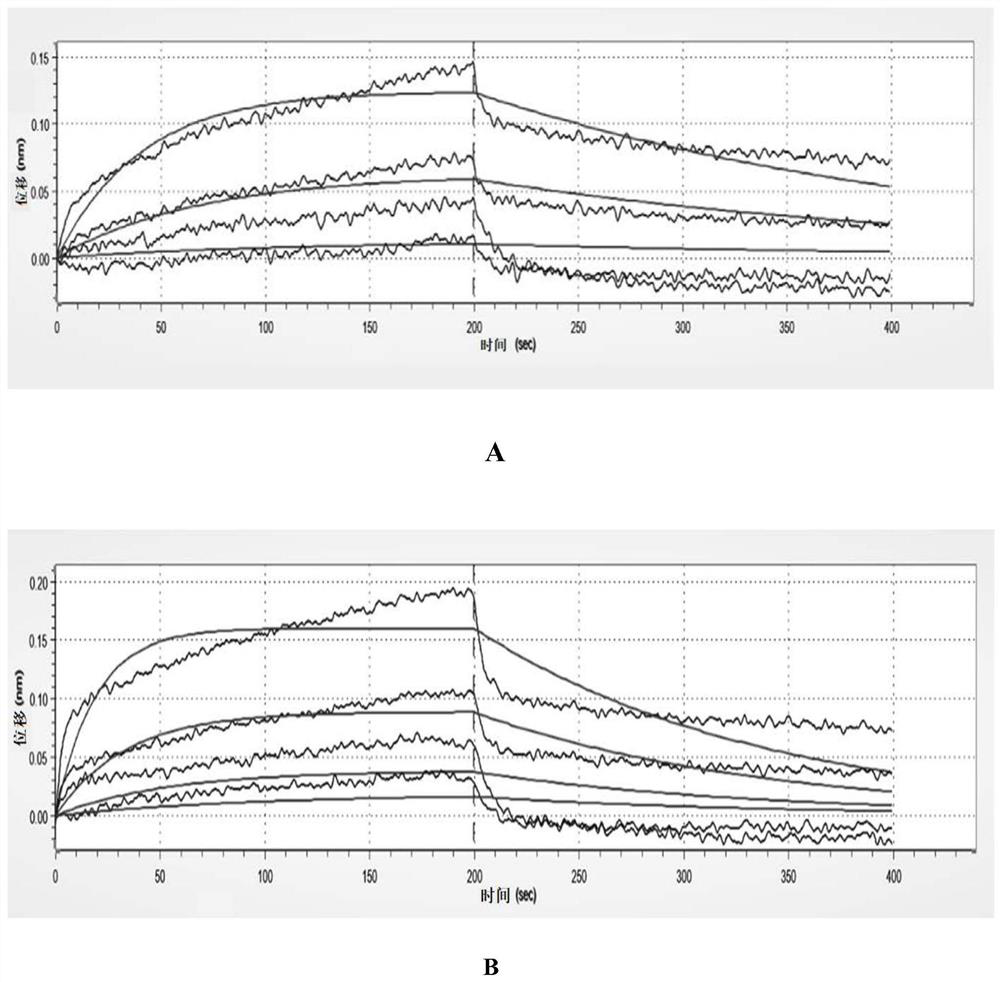
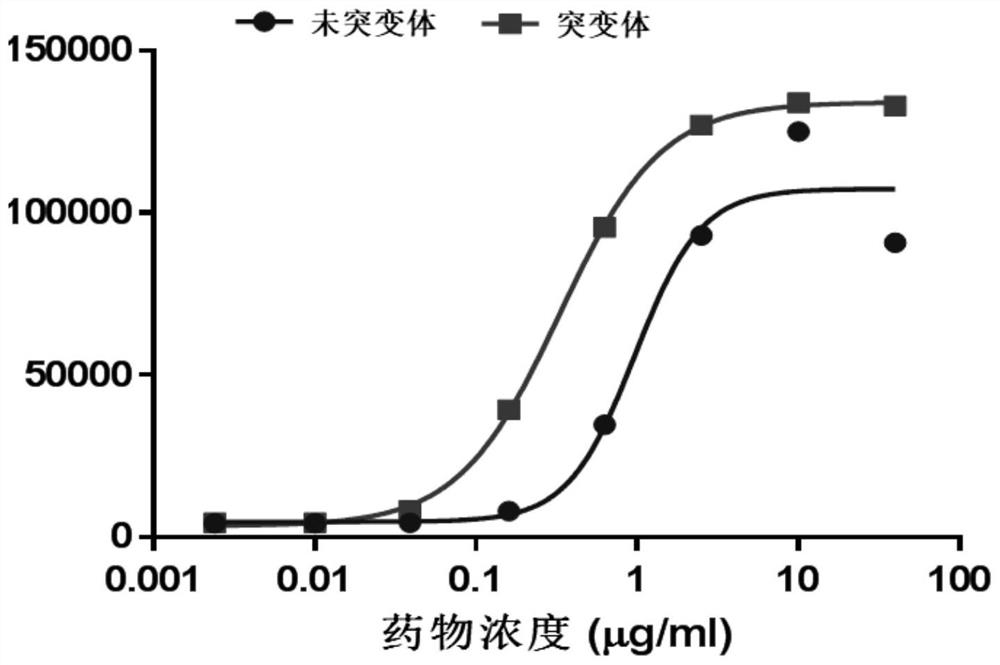
[0058] The gator non-labeled biomolecular analyzer (probelife) of Suzhou Jingyuan Laboratory was used to detect the binding affinity of interferon-κ and its receptor according to the operation steps of its instruction manual: firstly, the purified interferon-κ was diluted in multiples, A total of 5 concentrations, and then let the anti-huFc probe bind to the interferon receptor first, and then react with different concentrations of interferon kappa. At this time, the receptor binds to the interferon-κ protein to form a dynamic curve, and then the reacted The probe is placed in the dissociation buffer, at this time the receptor and the interferon-κ protein will dissociate slowly to form a dynamic curve, and finally the affinity is calculated according to the curve and concentration. The specific results are shown in Table 1 and figure 1 A-B are shown. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com