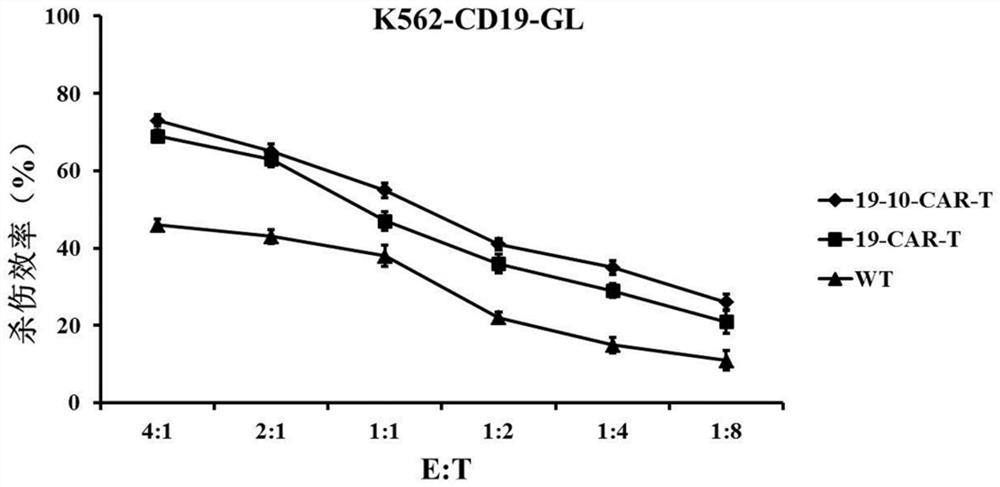
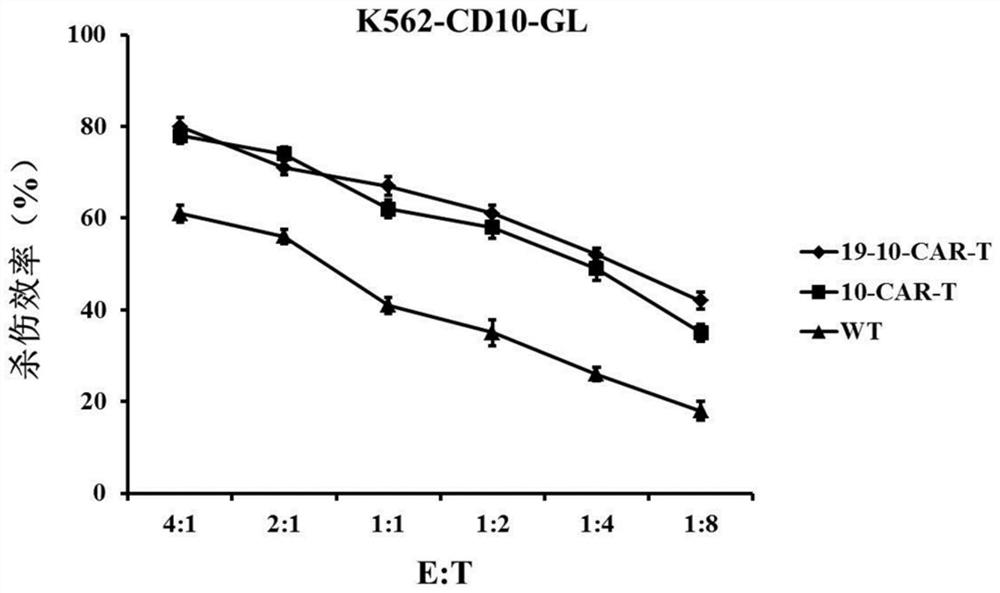
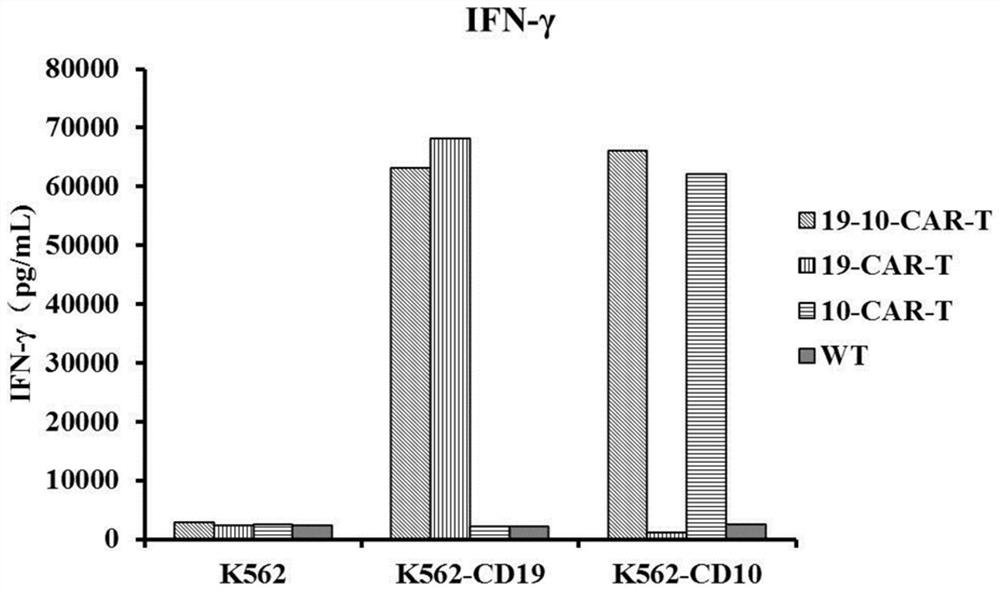
CD19 and CD10 dual-target chimeric antigen receptor and its application
A chimeric antigen receptor and single-chain antibody technology, applied in the field of biomedicine, can solve problems such as poor treatment effect and tumor recurrence, and achieve the effects of avoiding immune escape, efficient targeting, and avoiding target escape phenomenon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 Construction of CAR Molecular Carrier
[0054] In this example, anti-CD19 and anti-CD10 dual-target chimeric antigen receptor 19-10-CAR was constructed. The amino acid sequence is shown in SEQ ID NO:3, and the coding gene is shown in SEQ ID NO:6;
[0055] First, the whole gene is synthesized as SEQ ID NO: 6, and EcoRI and BamHI restriction sites and their protective bases are added at both ends;
[0056] Double-digest the coding gene with restriction endonucleases EcoRI and BamHI, incubate in a water bath at 37°C for 30 minutes, and use 1.5% agarose gel electrophoresis to recover the digested product containing sticky ends;
[0057] The digested product was ligated into the linearized pLVX-EF1-MCS plasmid (containing cohesive ends) that had been digested with EcoRI and BamHI, and the ligation system was shown in Table 1 to obtain a CAR containing dual targets targeting CD19 and CD10. Lentiviral vector encoding the gene.
[0058] Table 1
[0059] c...
Embodiment 2
[0061] Example 2 Lentiviral packaging
[0062] In this example, the lentiviral vector constructed in Example 1 is used for lentiviral packaging, using a four-plasmid system, and the steps are as follows:
[0063] Mix the helper plasmids gag / pol, Rev and VSV-G with the recombinant vector in proportion, add it to a certain volume of serum-free DMEM, mix it and let it stand for 15 minutes; add the above mixture to the cell culture flask lined with 293T cells , mixed gently, at 37°C, 5% CO 2 Cultivate in the cell incubator for 6 hours; after 6 hours, replace the fresh medium, continue to cultivate, and add 10mM sodium butyrate solution; after 72 hours, collect the lentivirus culture supernatant for purification and detection.
[0064] Recombinant vectors include lentiviral vectors containing genes encoding CARs targeting both CD19 and CD10, lentiviral vectors containing genes encoding CARs targeting CD19 single targets, and lentiviral vectors containing genes encoding CARs target...
Embodiment 3
[0065] Example 3 T cell activation and lentiviral transfection
[0066] Peripheral blood mononuclear cells (PBMC) were separated from whole blood using Ficoll density gradient centrifugation kit (GE Company), and after red blood cells were removed, T cells were sorted out using MACS Pan-T magnetic beads;
[0067] The sorted T cells were diluted with medium (AIM-V medium + 5% FBS + penicillin 100 U / mL + streptomycin 0.1 mg / mL) to a cell concentration of 2.5×10 6 pcs / mL for use;
[0068] CD2 / CD3 / CD28 T cell activation expansion kit (Miltenyi Company) was used to activate T cells, that is, the coated magnetic beads were mixed with T cells at a ratio of 1:2, and the final density of T cells was 5×10 6 piece / mL / cm 2 , after mixing, place at 37°C, 5% CO 2 The incubator was stimulated for 48 hours;
[0069] After T cells were activated for 48 hours, the beads were demagnetic, centrifuged at 300 g for 5 min, and the supernatant was removed. T cells were resuspended in fresh medium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com