Buffered formulations of bevacizumab for use of treating diseases
A preparation and antibody technology, which can be applied to sensory diseases, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve problems such as the reduction of antibody efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Method used
Image
Examples
Embodiment 1
[0184] Reality Example 1 - Materials and methods
[0185] Introduction. The antibody ONS-5010 represents a biosimilar of bevacizumab and has been reformulated for enhanced storage stability. The buffered formulation is believed to reduce antibody aggregation, at least during long-term storage. It is believed that the buffer formulation can reduce non-covalent and covalent dimerization of the bevacizumab molecule. Bevacizumab, marketed as Atorvastatin® (Genentech, Inc.), was formulated in sodium phosphate buffer, which included trehalose as a stabilizer, and included a mild surfactant and an acidic pH of 6.2. The protocol described below included the development of reformulating bevacizumab for enhanced colloidal stability. Significant improvements in stability (especially in terms of reducing aggregation) were achieved by varying the buffer and pH.
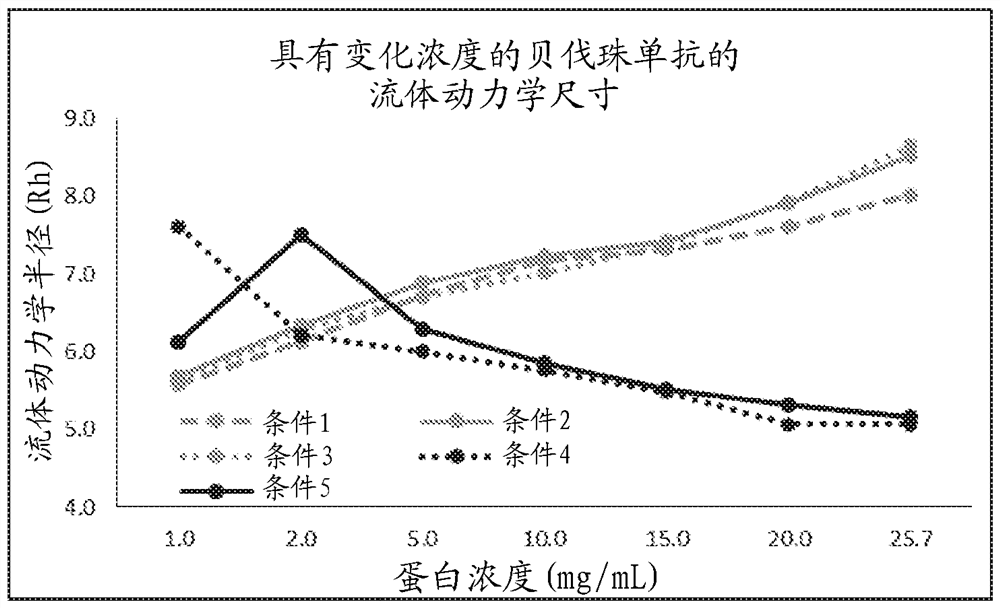
[0186] Dynamic Light Scattering (DLS). DLS test method using Wyatt DynaPro TM Plate reader to provide information on prote...
Embodiment 2
[0197] Example 2 - Effect of Buffer, Stabilizer and pH on Bevacizumab Conformation and Colloidal Stability
[0198]Initial experiments evaluated the effect of buffer components on the conformation and colloidal stability of bevacizumab. Citrate, phosphate and acetate buffers were determined to be ideal for the stability of bevacizumab. Furthermore, these buffers alone showed protection against aggregation of bevacizumab induced by heat- or shaking-related stress. Further experiments evaluated whether combinations of these buffers (citrate, phosphate, and acetate) exhibited superior stabilization. Citrate phosphate buffer produced significantly lower aggregates (including covalent dimers) and lower charged species.
[0199] The effect of trehalose stabilizers in 50 mM sodium phosphate buffer was compared to alternative stabilizers including sucrose, sorbitol, mannitol and glycine. The conformational stability of the antibody in different stabilizing buffer compositions wa...
Embodiment 3
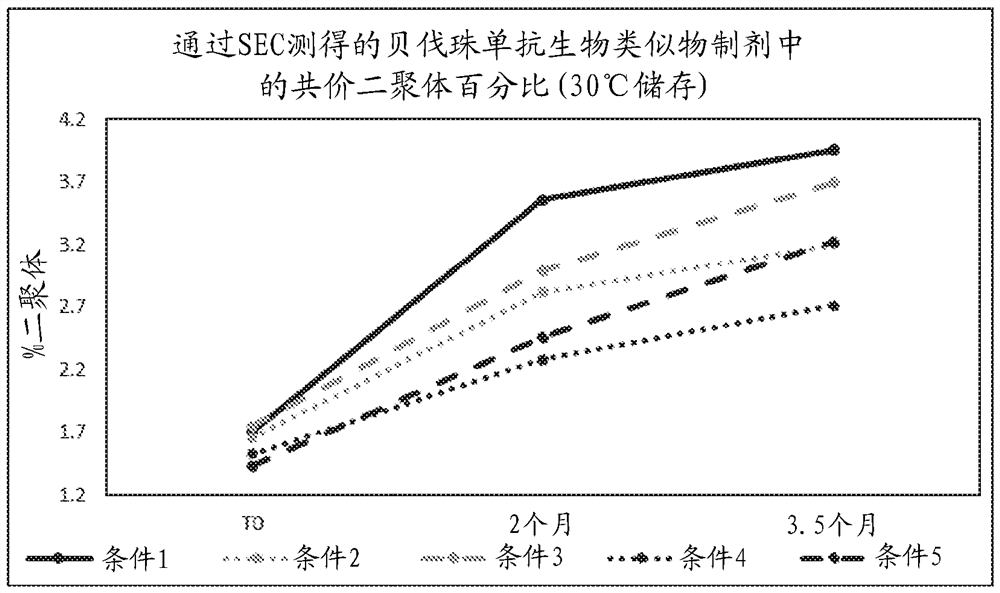
[0213] Example 3 - Storage Stability in Citrate Phosphate Buffered Trehalose and Acetate Sucrose Formulations
[0214] Four buffered formulations were selected to evaluate the long-term storage stability of the bevacizumab molecule over 18 months. These formulations were performed in parallel with the bevacizumab matching / reference formulation (Condition 1). Storage conditions were as follows: approximately 25 mg / ml (neat) or diluted antibody; storage at 5°C, 30°C, or 37°C; shaking at room temperature at 150 RPM; and freeze / thaw (20°C to room temperature for three cycle). The formulations tested are listed below:
[0215] Condition 1: Bevacizumab (Arvastatin®) matching
[0216] 50 mM sodium phosphate
[0217] 159 mM Trehalose
[0218] 0.04% Polysorbate 20
[0219] pH 6.20
[0220] Supplement to appropriate amount with sterile water for injection
[0221] Condition 2: Bevacizumab Citrate Phosphate, pH 5.8
[0222] 50 mM citrate phosphate
[0223] 159 mM Trehalos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com