Virus and antigen purification and conjugation
A virus and antigen technology, applied in the field of virus and antigen purification and coupling, can solve the problem of insufficient removal of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
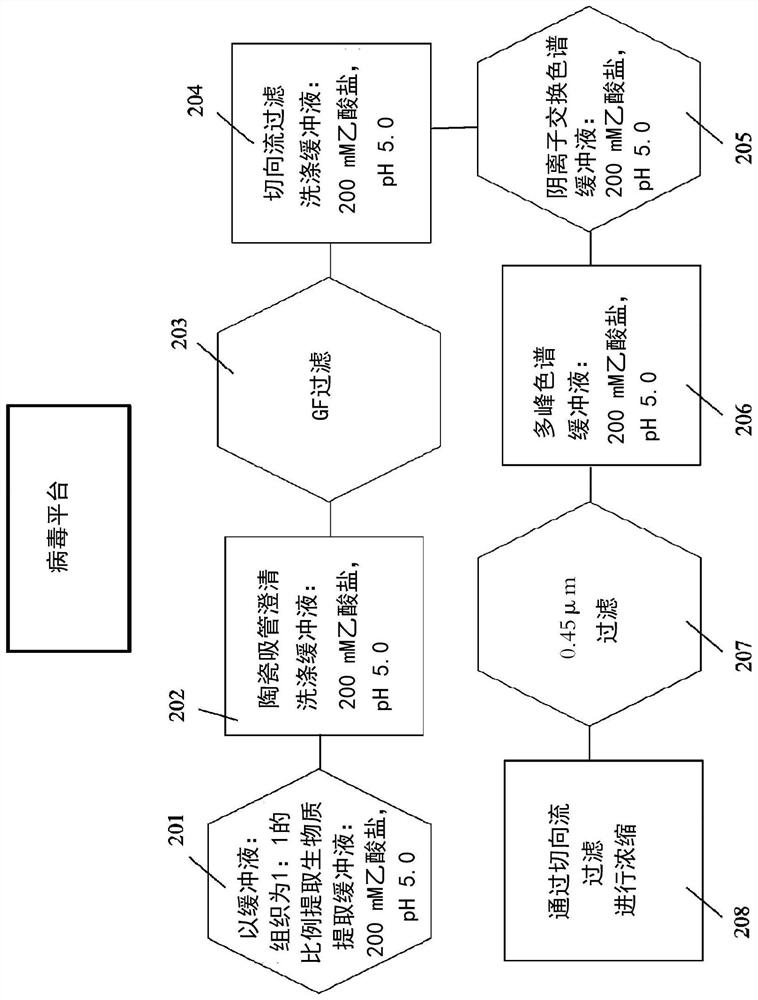
[0095] Embodiment 1—purification of icosahedral red clover mosaic virus
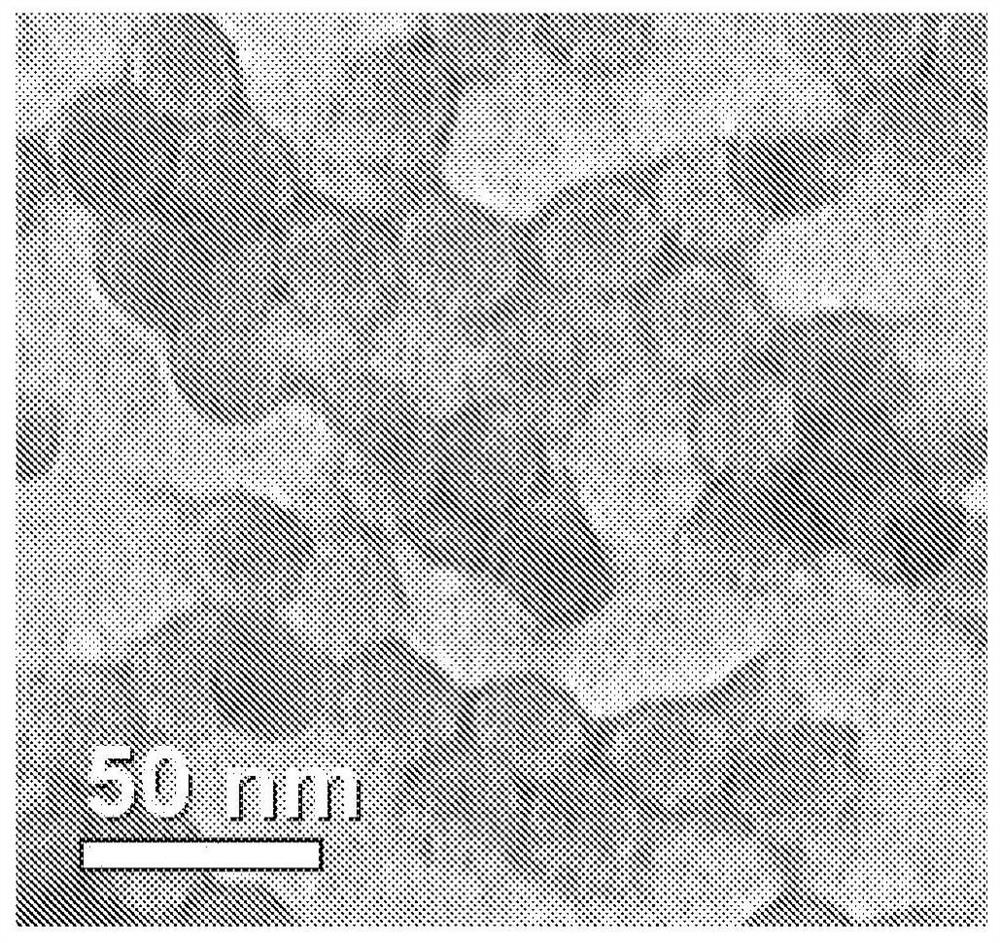
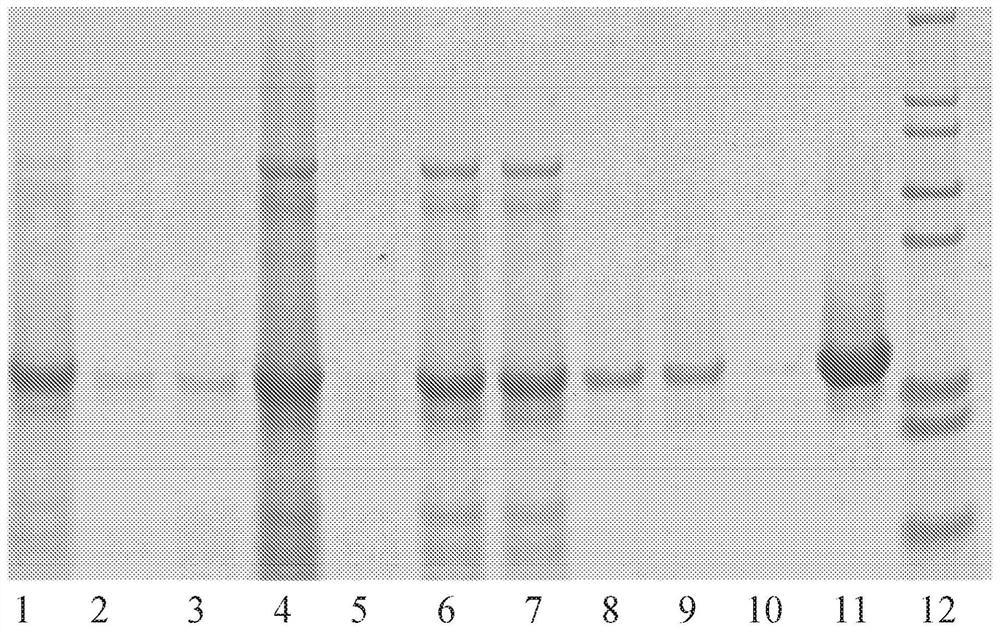
[0096] As a known technique for detecting various proteins in a mixture, image 3 The Western Blot provided in , which shows figure 2 The successful purification of the icosahedral red clover mosaic virus is shown. Similarly, Figure 5 Western blot in Figure 4 The successful purification of the icosahedral red clover mosaic virus is shown. Both viruses were purified according to the embodiments described herein. The target protein is extracted from the tissue according to known detection techniques. The proteins of the sample are then separated using gel electrophoresis according to their isoelectric point, molecular weight, charge, or various combinations of these factors. The samples are then loaded into individual lanes in the gel, with one lane reserved for a "ladder" containing a mixture of known proteins of defined molecular weight. For example in image 3 In , lane 12 serves as a ladde...
Embodiment 2
[0099] Example 2—Purification of Rod-Shaped TMV
[0100] Image 6 shows purified rod-shaped TMV, Figure 7 A viral purification platform for TMV to achieve this purification is shown within the scope of the various embodiments and alternatives disclosed herein. and image 3 with Figure 5 similar, Figure 7 Shows the purity of the virus product after each step of the virus purification platform is completed. After the final purification step, the resulting product is a highly purified virus product, with Figure 7 The clearly visible bands in lane 13 are consistent.
[0101] Thus, the virus purification platform of the present invention has successfully purified every virus for which the inventors have applied these methods, including icosahedral and baculoviruses, and the platform is expected to reproducibly and consistently purify almost Viruses of any type, if not all types.
[0102] Production and purification of recombinant antigens
[0103] Table 2 and Fig...
Embodiment 3、4、5 and 6
[0116] Examples 3, 4, 5 and 6—H5 rHA, H7 rhA, WNV rDIII and LFV rGP1 / 2
[0117] Such as Figure 12 As shown, antigen purification platforms according to various embodiments and alternatives have successfully purified H5rHA, H7 rhA, WNV rDIII and LFV rGP1 / 2. Figure 12 Contains two images taken from the conclusion of the antigen purification platform: the left image contains an SDS Page gel indicating the purity of the viral vector TMV NtK (where NtK is an abbreviation for N-terminal lysine) and influenza antigens, and the right image contains a Western blot , indicating immunoreactivity to West Nile and Lassa fever antigens. Such as Figure 12 Each antigen product is highly pure, as shown by the clearly visible bands in . Thus, the antigen purification platform according to various embodiments and alternatives consistently purifies each type of antigen on a commercial scale in a manner compliant with cGMP regulations. In the same way, the platform promises to rep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Radius | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com