Preparation method of olanzapine dihydroxynaphthoate sustained-release particle preparation spray
A technology of olanzapine pamoate and slow-release microparticles, which is applied in the direction of pharmaceutical formulas, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., and can solve the problem of low incidence of extrapyramidal reactions, Application restrictions, toxic and side effects, etc., to achieve the effect of improving compliance, easy operation, and eliminating toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
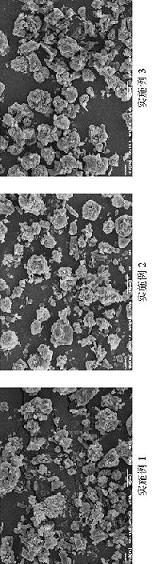
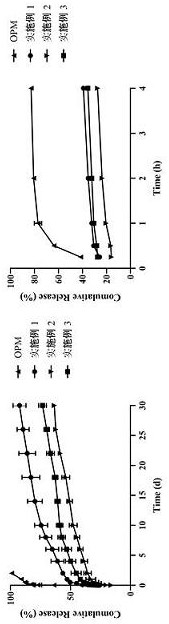
Embodiment 1
[0018] 1) Weigh 400 mg of PLGA 7525 7A, dissolve in 50 mL of dichloromethane, and dissolve with ultrasonic stirring;
[0019] 2) Weigh 600 mg of olanzapine pamoate powder, add it to the above solution, and ultrasonically stir to suspend, and set aside;
[0020] 3) The solution is spray-dried with a laboratory-scale BUCHI B-290 spray dryer with a 60 cm long drying column and a nozzle with a diameter of 0.7 mm. The conditions of the dryer are as follows: inlet temperature = 45 ° C, outlet temperature = 32 ℃, air flow = 50 cubic meters; collected after drying.
Embodiment 2
[0022] 1) Weigh 500 mg of PLGA 7525 7A, dissolve in 50 mL of dichloromethane, and dissolve with ultrasonic stirring;
[0023] 2) Weigh 500 mg of olanzapine pamoate powder, add it to the above solution, and ultrasonically stir to suspend, and set aside;
[0024] 3) The solution is spray-dried with a laboratory-scale BUCHI B-290 spray dryer with a 60 cm long drying column and a nozzle with a diameter of 0.7 mm. The conditions of the dryer are as follows: inlet temperature = 45 ° C, outlet temperature = 32 ℃, air flow = 50 cubic meters; collected after drying.
Embodiment 3
[0026] 1) Weigh 600 mg of PLGA 7525 7A, dissolve in 50 mL of dichloromethane, and dissolve with ultrasonic stirring;
[0027] 2) Weigh 400 mg of olanzapine pamoate powder, add it to the above solution, and ultrasonically stir to suspend, and set aside;
[0028] 3) The solution is spray-dried with a laboratory-scale BUCHI B-290 spray dryer with a 60 cm long drying column and a nozzle with a diameter of 0.7 mm. The conditions of the dryer are as follows: inlet temperature = 45 ° C, outlet temperature = 32 ℃, air flow = 50 cubic meters; collected after drying.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com