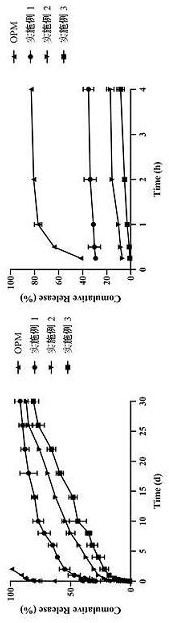
Olanzapine-pamoate sustained-release microparticle preparation and preparation method therefor
A technology for olanzapine pamoate and sustained-release microparticles, which is applied in the field of olanzapine pamoate sustained-release microparticle preparations and its preparation, and can solve the problems of low incidence of extrapyramidal reactions, application restrictions, and toxicity. side effects and other issues, to achieve the effect of improving compliance, easy operation, and eliminating side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 1) Weigh 50 mg of PLGA 7525 7A, dissolve in 5 mL of dichloromethane, and dissolve with ultrasonic stirring;
[0017] 2) Weigh 150 mg of olanzapine pamoate powder, add it to the above solution, stir and suspend with ultrasonic, and set aside;
[0018] 3) Measure 20 mL of paraffin in a 100 mL beaker, set the homogenizer to 10000 rpm, and mix the above suspension with
[0019] Inject the 20 mL syringe into the beaker within 30 s and homogenize for 3 min;
[0020] 4) Magnetically stirred for 4 h, dichloromethane was volatilized, collected by suction filtration, washed three times with 120 mL of n-hexane, and obtained.
Embodiment 2
[0022] 1) Weigh 75 mg of PLGA 7525 7A, dissolve in 5 mL of dichloromethane, and dissolve with ultrasonic stirring;
[0023] 2) Weigh 150 mg of olanzapine pamoate powder, add it to the above solution, stir and suspend with ultrasonic, and set aside;
[0024] 3) Measure 20 mL of paraffin into a 100 mL beaker, set the homogenizer at 10000 rpm, inject the above suspension into the beaker with a 20 mL syringe within 30 s, and homogenize for 3 min;
[0025] 4) Magnetically stirred for 4 h, dichloromethane was volatilized, collected by suction filtration, washed three times with 120 mL of n-hexane, and obtained.
Embodiment 3
[0027] Olanzapine pamoate PLA sustained-release microparticles prepared by emulsification solvent evaporation method:
[0028] 1) Weigh 100 mg of PLGA 7525 7A, dissolve in 5 mL of dichloromethane, and dissolve with ultrasonic stirring;
[0029] 2) Weigh 150 mg of olanzapine pamoate powder, add it to the above solution, stir and suspend with ultrasonic, and set aside;
[0030] 3) Measure 20 mL of paraffin into a 100 mL beaker, set the homogenizer at 10000 rpm, inject the above suspension into the beaker with a 20 mL syringe within 30 s, and homogenize for 3 min;
[0031] 4) Magnetically stirred for 4 h, dichloromethane was volatilized, collected by suction filtration, washed three times with 120 mL of n-hexane, and obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com