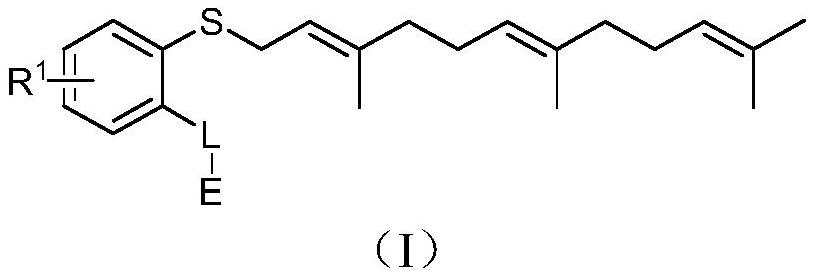
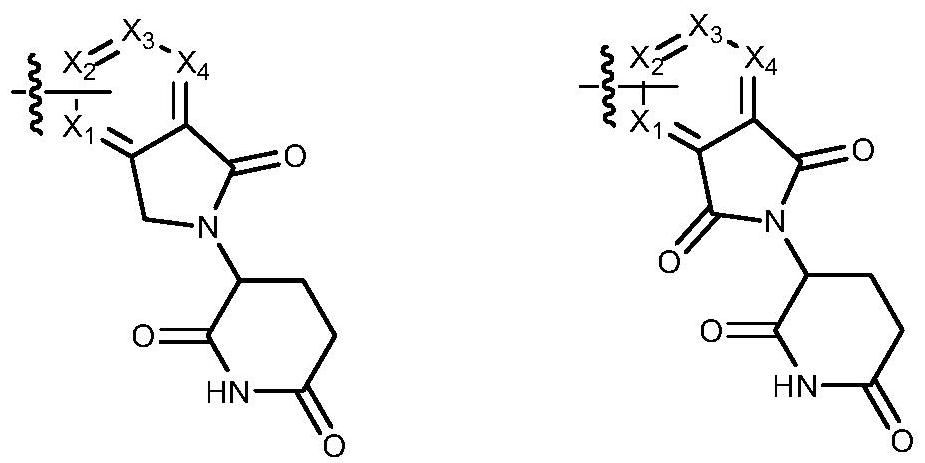
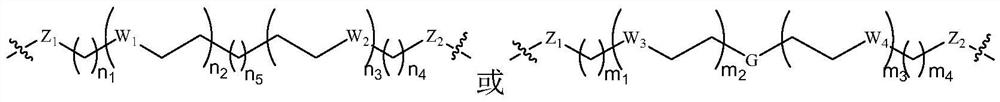
Protein degradation targeting chimera compound, preparation method and medical application thereof
A compound and pharmaceutical technology, applied in the field of medicine, can solve problems such as long progression-free period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0218] Example 1N-(2-{2-[2-(2,6-dioxo-piperidin-3-yl)-1,3-dioxo-2,3-dihydro-1H-isoindole -4-ylamino]-ethoxy}-ethyl)-2-(3,7,11-trimethyldodeca-2,6,10-trienylthio)-benzamide (1 ) preparation
[0219]
[0220] Step 1: Preparation of 2-(2,6-dioxo-piperidin-3-yl)-4-fluoro-isoindole-1,3-dione (1a)
[0221] At 140°C, 3-fluorophthalic anhydride (28g, 168mmol), 3-amino-2,6-piperidinedione hydrochloride (27.74g, 168mmol) and sodium acetate (16.5g, 201.6mml) were dissolved in 420ml of acetic acid Stirring for 14 hours. The reaction solution was cooled to room temperature, and a solid precipitated out, which was filtered, and the filter cake was washed with methyl tert-butyl ether. Blast drying (60° C.) for 8 hours gave 38 g of white solid, which was directly used in the next reaction without further purification.
[0222] Step 2: Preparation of 1-bromo-3,7,11-trimethyldodecane-2,6,10-triene (1b)
[0223] Dissolve 1-hydroxy-3,7,11-trimethyldodecane-2,6,10-triene (7.457g, 33.5mmol)...
Embodiment 2
[0236] Example 2N-(2-{2-[2-(2,6-dioxo-piperidin-3-yl)-1,3-dioxo-2,3-dihydro-1H-isoindole -5-ylamino]-ethoxy}-ethyl)-2-(3,7,11-trimethyldodeca-2,6,10-trienylthio)-benzamide (2 ) preparation
[0237]
[0238] The preparation method is the same as in Example 1, except that 3-fluorophthalic anhydride in step 1 is replaced with 4-fluorophthalic anhydride to obtain N-(2-{2-[2-(2,6-dioxo-piperidine -3-yl)-1,3-dioxo-2,3-dihydro-1H-isoindol-5-ylamino]-ethoxy}-ethyl)-2-(3,7,11 - Trimethyldodeca-2,6,10-trienylthio)-benzamide.
[0239] 1 H-NMR (400MHz, DMSO-d 6 )δ: 11.05(s, 1H), 8.29(d, J=4.8Hz, 1H), 8.19(d, J=8.0Hz, 1H), 7.55(d, J=8.4Hz, 1H), 7.48-7.32( m,5H),7.26(d,J=8.0Hz,1H),7.22(t,J=7.6Hz,1H),7.00(s,1H),6.89(d,J=8.4Hz,1H),5.05- 5.00(dd,J=5.2,12.8Hz,2H),4.66(t,J=5.2Hz,1H),3.62(t,J=4.4Hz,2H),3.55(t,J=4.4Hz,2H), 3.49-3.45(m,2H),3.39-3.33(m,4H),2.95-2.84(m,3H),2.59-2.50(m,3H),2.01-1.86(m,8H),1.70-1.54(m ,12H).
[0240] LC-MS(ESI):701.3(M+H) + .
Embodiment 3
[0241]Example 3 2-{2-[2-(2,6-dioxo-piperidin-3-yl)-1,3-dioxo-2,3-dihydro-1H-isoindole-4 -ylamino]-ethoxy}-ethyl 2-(3,7,11-trimethyl-dodeca-2,6,10-trienylthio)-benzoate (3) preparation
[0242]
[0243] Step 1: 2-(2,6-Dioxo-piperidin-3-yl)-4-[2-(2-hydroxy-ethoxy)-ethylamino]-isoindole-1,3- Preparation of diketone (3a)
[0244] Compound 1a (2.0g, 7.2mmol), diglycolamine (1.14g, 10.9mmol) and diisopropylethylamine (1.86g, 14.4mmol) were dissolved in DMF (10mL), and the reaction system was heated to Stir overnight at 90°C. The reaction solution was lowered to room temperature, then poured into 60ml of water, extracted with ethyl acetate 150ml×2, the organic phase was washed with saturated brine 100ml×2, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure. The residue was purified by column chromatography (eluent: dichloromethane / ammonia methanol=100:1-50:1) to obtain 880 mg of the product as a yellow solid.
[0245] Step ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com