Anti-abeta antibodies and uses thereof
A technology of antibody and β protein, applied in the direction of antibody, anti-animal/human immunoglobulin, immunoglobulin, etc., can solve the problem of lack of effective treatment for AD
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Generation of seven antibody clones against Aβ from fusion tumors.
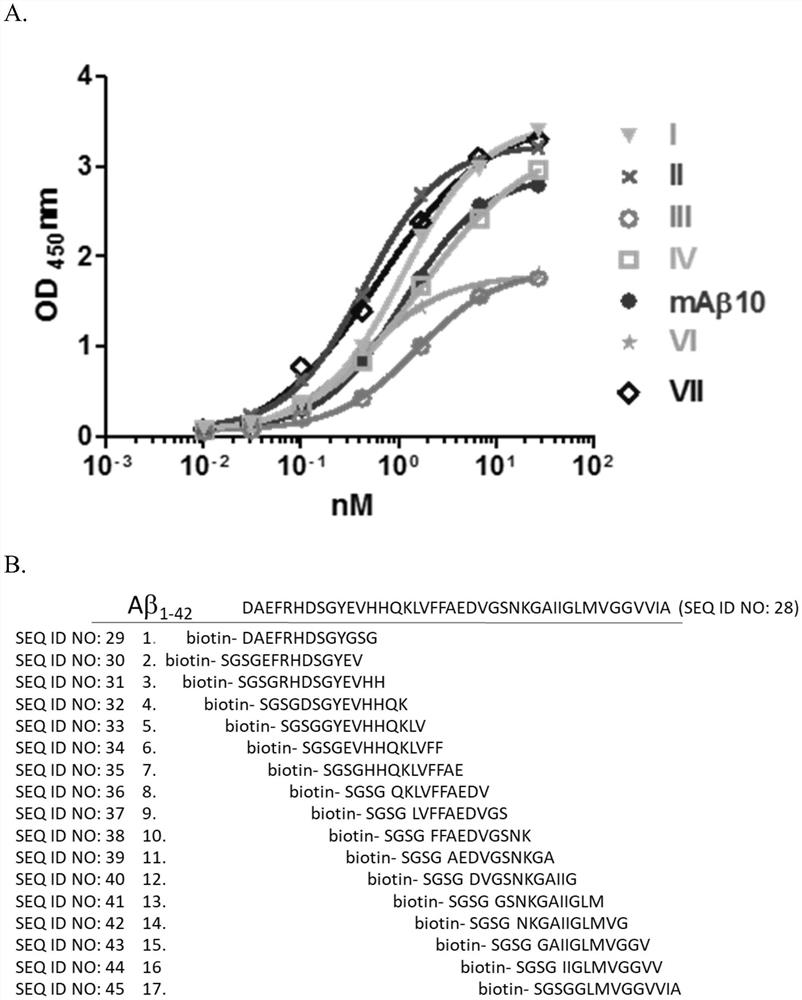
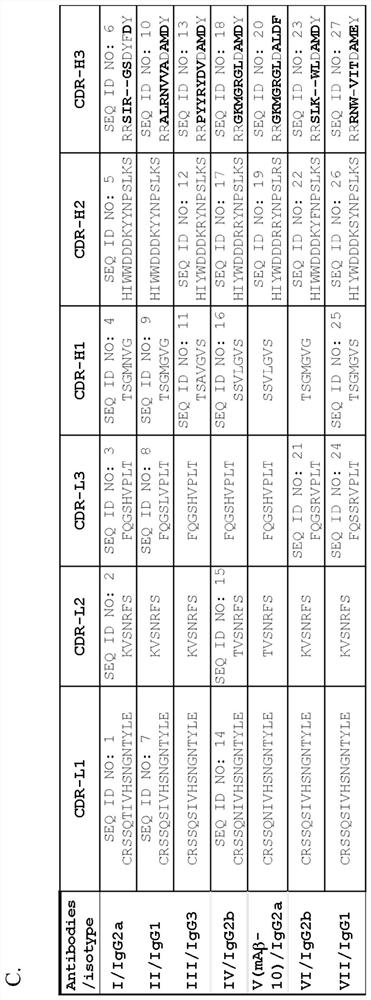
[0041] To generate fusionomas, mice were immunized with oligomeric Aβ (oAβ). synthesized Aβ 1–42 Dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) and evaporated. Dried membranes were redissolved in 1XPBS, followed by centrifugation to remove fibrillar and aggregated A[beta]. The prepared oAβ was characterized by western blotting using a commercially available Aβ antibody 6E10. The results showed that the prepared oAβ oligomer constituted a mixture of Aβ, including monomer, dimer and oAβ in various structural orders, with a molecular weight range of 37-250kDa. The prepared oAβ oligomers were stored at -80°C until submitted to LTK Biolaboratories for immunization to generate single fusion tumors. By ELISA ( figure 1 A) Antibodies were analyzed for Aβ binding affinity by immunohistochemistry (data not shown). Create an epitope map of your antibody. please see figure 1 b. Seven dif...
Embodiment 2
[0042] Example 2: Production of Chimeric Antibodies
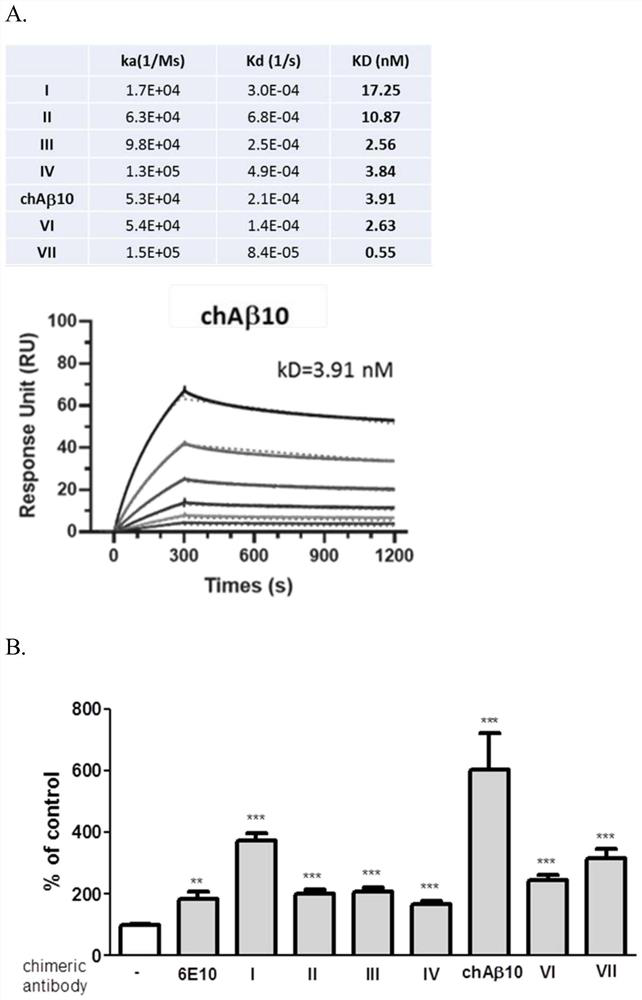
[0043] The mouse IgG variable regions were inserted into the human IgG1 backbone to generate chimeric antibodies. The Aβ-binding affinities of the seven chimeric antibodies were evaluated by surface plasmon resonance (SPR) with Biacore. please see figure 2 a. In vitro experiments were performed to test the effect of the antibody on enhancing the phagocytosis of Aβ by microglia. The data indicated that all antibodies enhanced Aβ uptake by microglia, with the lead antibody appearing to perform best in the assay. please see figure 2 b.
Embodiment 3
[0044] Example 3: Humanization of mAβ-10
[0045] A humanized version of the lead antibody (hzAβ-10) was constructed from a chimeric antibody using a human IgG1 framework. Analysis of protein solubility using fast protein liquid chromatography (FPLC) ( image 3 A), and by performing immunofluorescence histochemistry on APP / PS1 mouse brain sections (data not shown), ELISA ( image 3 B) and SPR ( image 3 C) Detection of A binding features. The data show that hzAβ-10 exhibits the special properties of excellent Aβ binding affinity and extremely high protein solubility, which is suitable for cell line development.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com