Antibody to be cross-linked to human and mouse sema3A, and use thereof
A semaphore, antibody technology, applied in the direction of antibodies, anti-animal/human immunoglobulin, etc., can solve the problems of high growth rate of cancer cells, poor prognosis of patients, and no anti-cancer drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
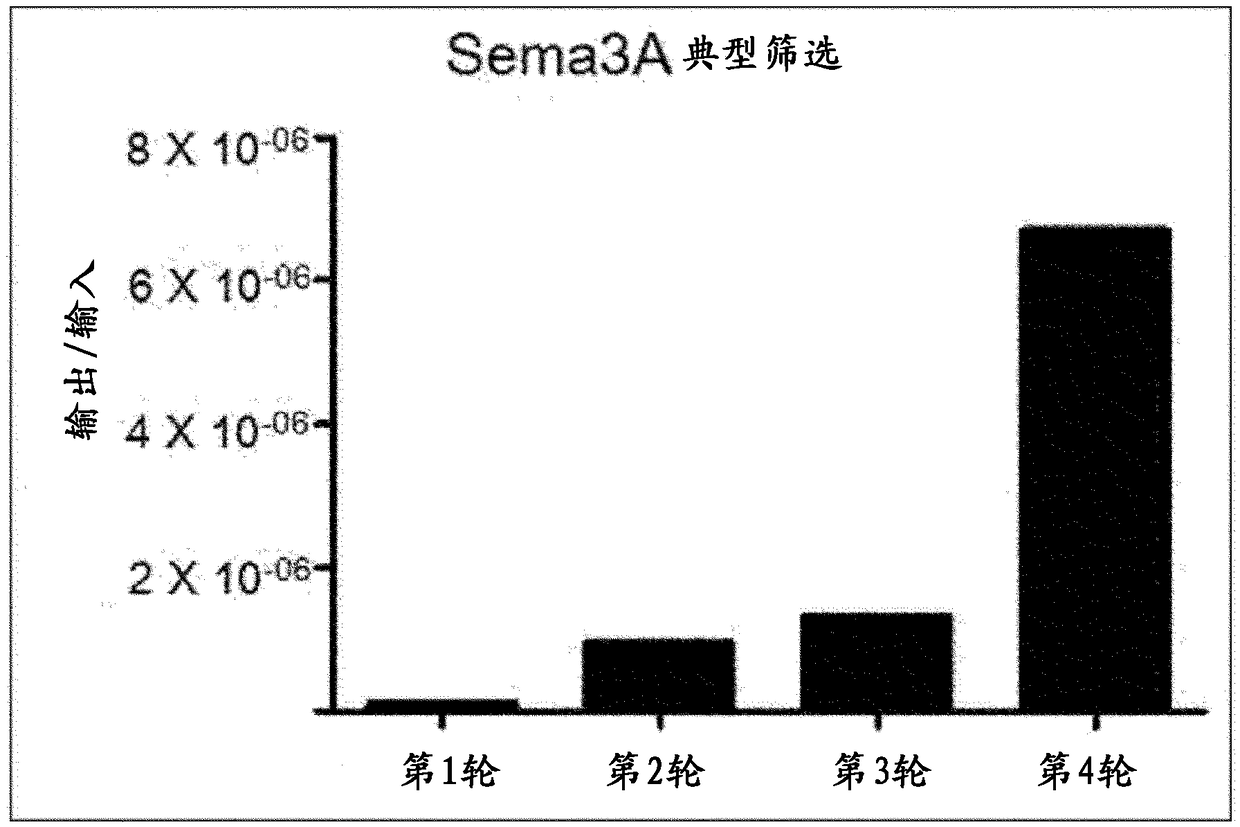
[0106] Example 1: Screening using human semaphorin 3A recombinant protein
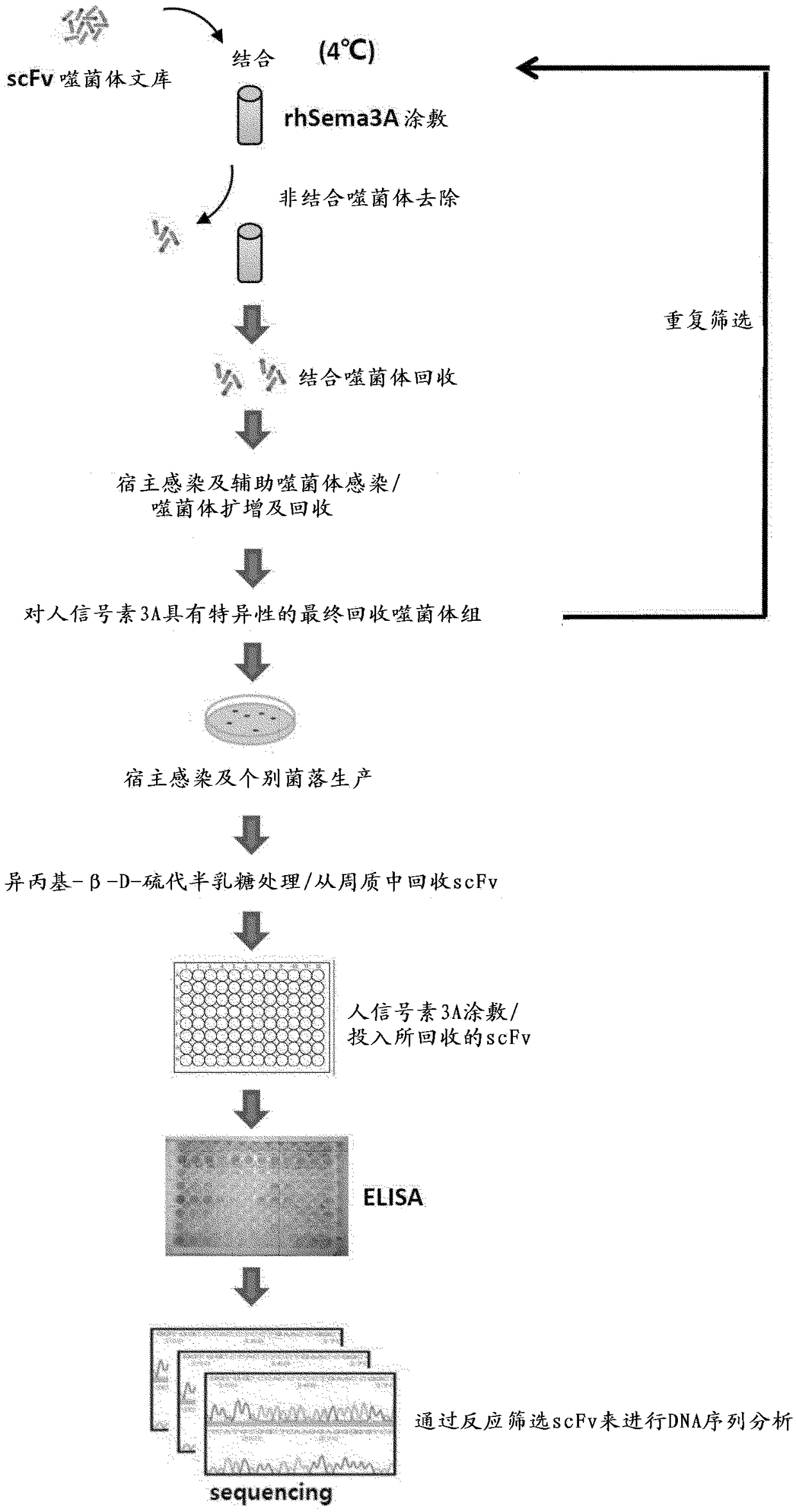
[0107] The synthetic scFv antibody fragment phage library (Yang et al., Mol. Cells. 27:225-235, 2009) made in the past was used to identify scFv antibody fragments that bind to human semaphorin 3A through phage display screening. The phage display screening process is shown in Figure 1.
[0108] In detail, in order to recover the phagemid vector introduced into the Escherichia coli host ER2537 in the form of phage, the lower library samples were cultured in 400 ml of medium (SB / ampicillin / 2% glucose) 2 hours. If the O.D600 absorbance reaches about 0.5 to 0.7, centrifuge at 5000g for 20 minutes to remove the supernatant, suspend it in 400ml of the second medium (SB / ampicillin), and then add 10 12 pfu (plaque forming unit) helper phage (VCSM13), incubated for 1 hour. After that, 70 μg / ml of kanamycin antibiotic (an antibiotic gene introduced into the helper phage) was added, and cultured overnight at ...
Embodiment 2
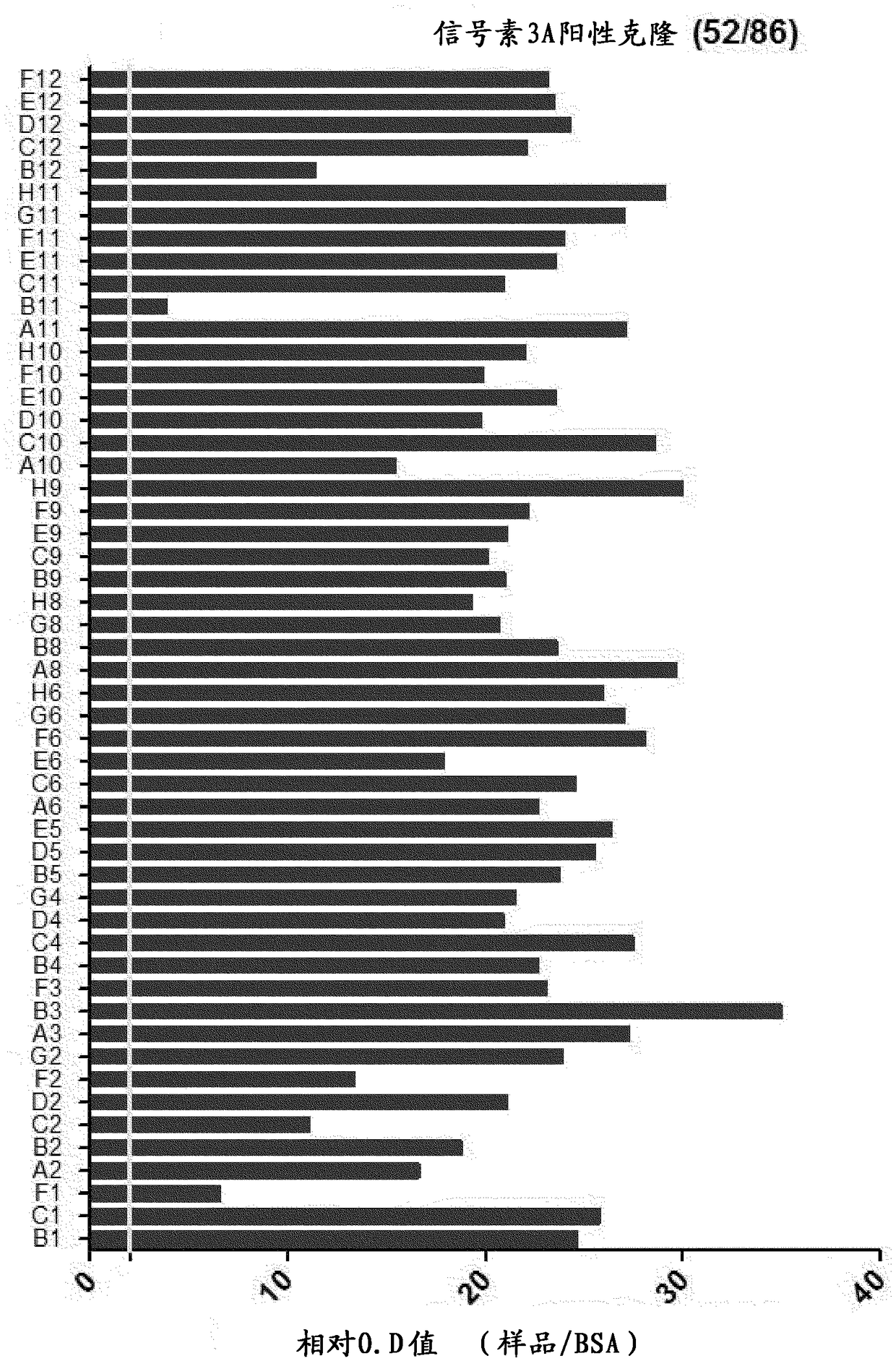
[0111] Example 2: ELISA and sequence analysis for subsequent screening of anti-semaphorin 3A scFv
[0112] The phage particles recovered in the fourth screening were confirmed as colonies in the medium by infecting the host cell ER2537. The colonies were collected and inoculated on a 96-well plate containing 200 μl of SB / ampicillin medium, and cultured at 37° C. for 2 to 3 hours. Then, in order to induce the expression of scFv-pⅢ protein, each well was treated with isopropyl-β-D-thiogalactopyranoside (IPTG, Isopropylβ-D-1-thiogalactopyranoside) at a final concentration of 1 mM at a temperature of 30°C. Incubate overnight. After centrifuging the cultured well plate at 3000 rpm to remove the supernatant, 40 μl of TES solution (20% w / v sucrose, 50 mM Tris, 1 mM EDTA, pH 8.0) and left at 4°C for 30 minutes to lyse the cells. Afterwards, 60 μl of 0.2X TES solution was treated and placed at 4° C. for 30 minutes. After osmotic pressure was used to decompose the cells, the plate wa...
Embodiment 3
[0114] Example 3: Production of anti-semaphorin 3A scFv protein and confirmation of semaphorin 3A binding ability
[0115] The basic structure of the phagemid can be found at Figure 6It was confirmed in the above procedure that in the case of the host cell ER2537 described in the procedure above, scFv could not be expressed alone due to suppression of the amber codon (amber codon (UAG)) located in front of phage pIII. Therefore, an expression strain (TOP10F') as a non-suppressor strain was used to transform the phagemid into the expression strain. Afterwards, it was confirmed by DNA sequence analysis that each phagemid did not produce a mutation and was introduced into the expression strain. After the expression strain was taken as a colony, it was inoculated in 3 ml of LB / ampicillin medium and incubated at a temperature of 37°C. Incubate overnight. After that, transfer 3 ml of the overnight culture solution to 400 ml of medium (SB / ampicillin) and culture at OD600 until it ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com