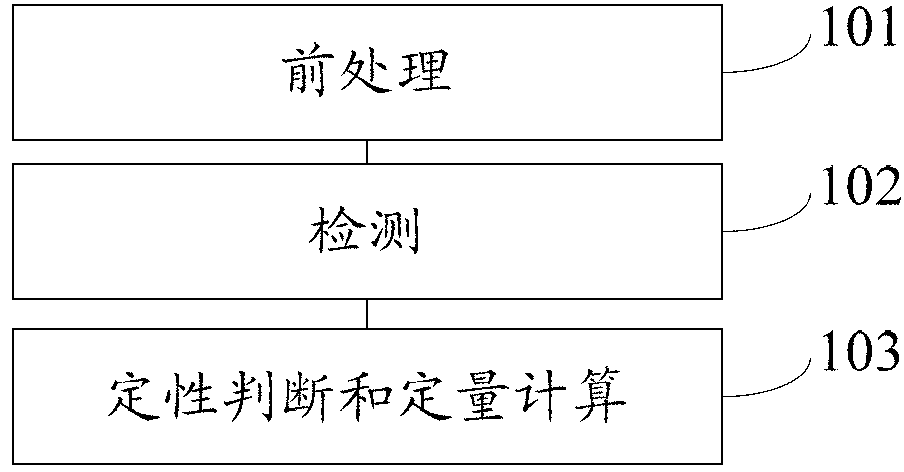
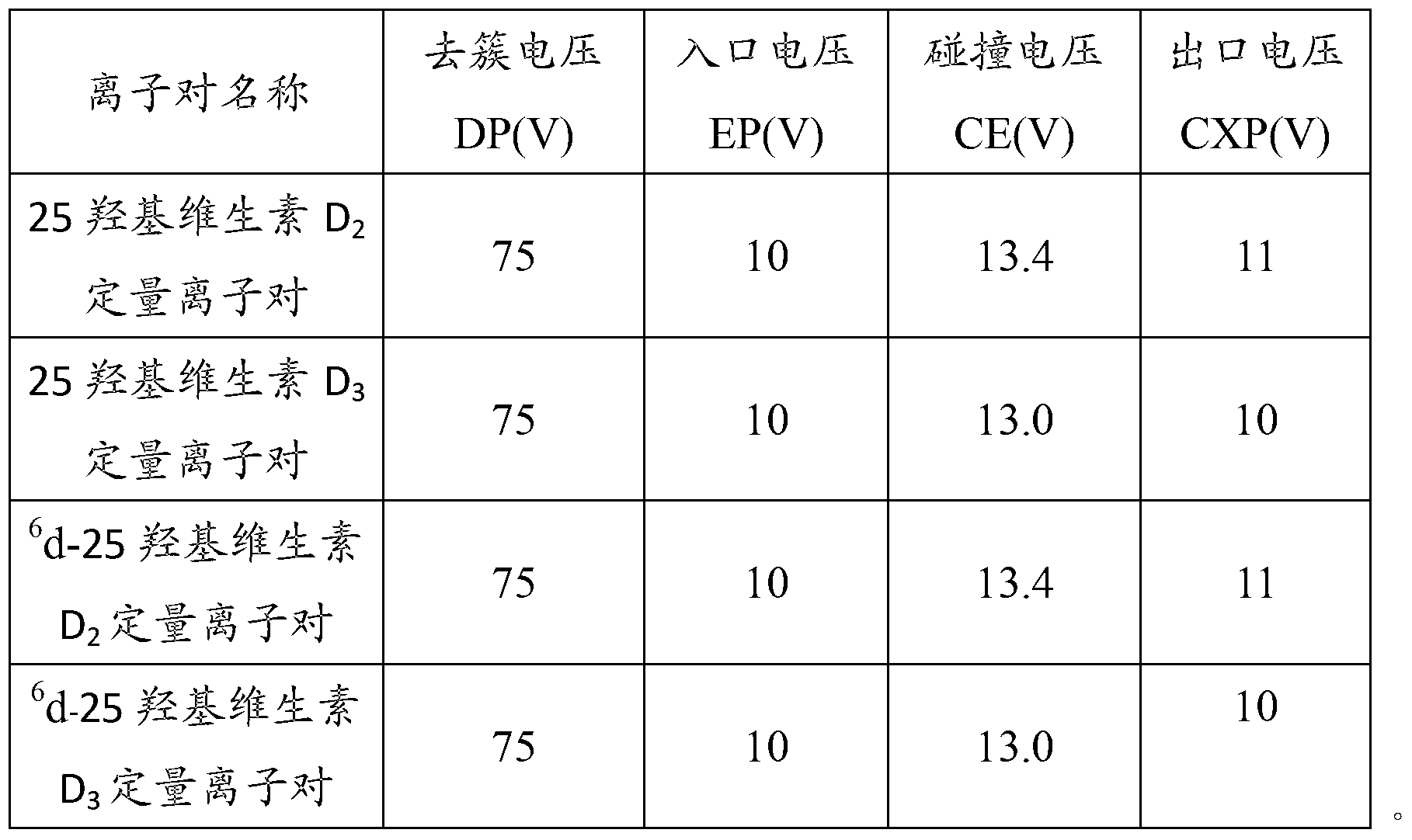
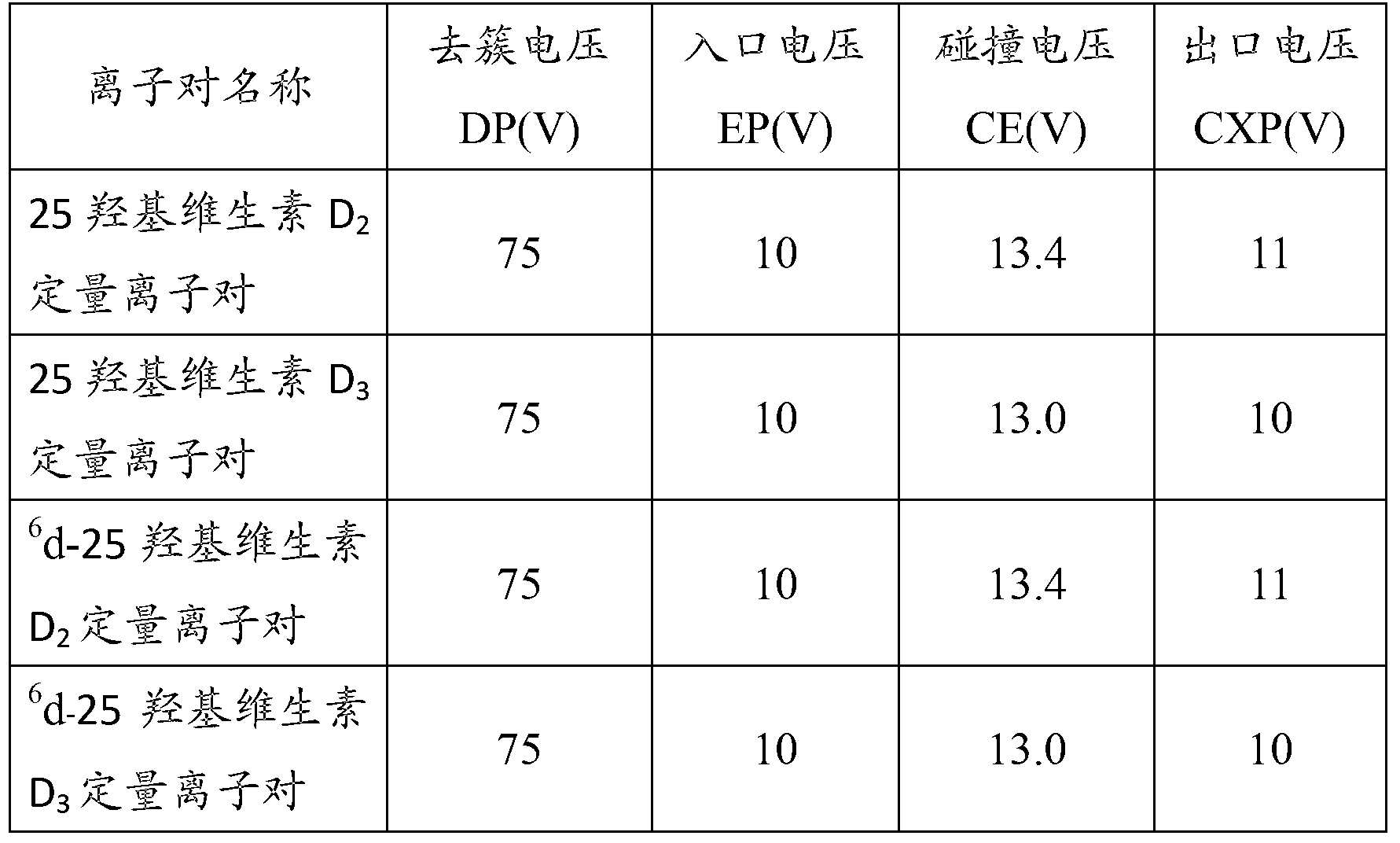
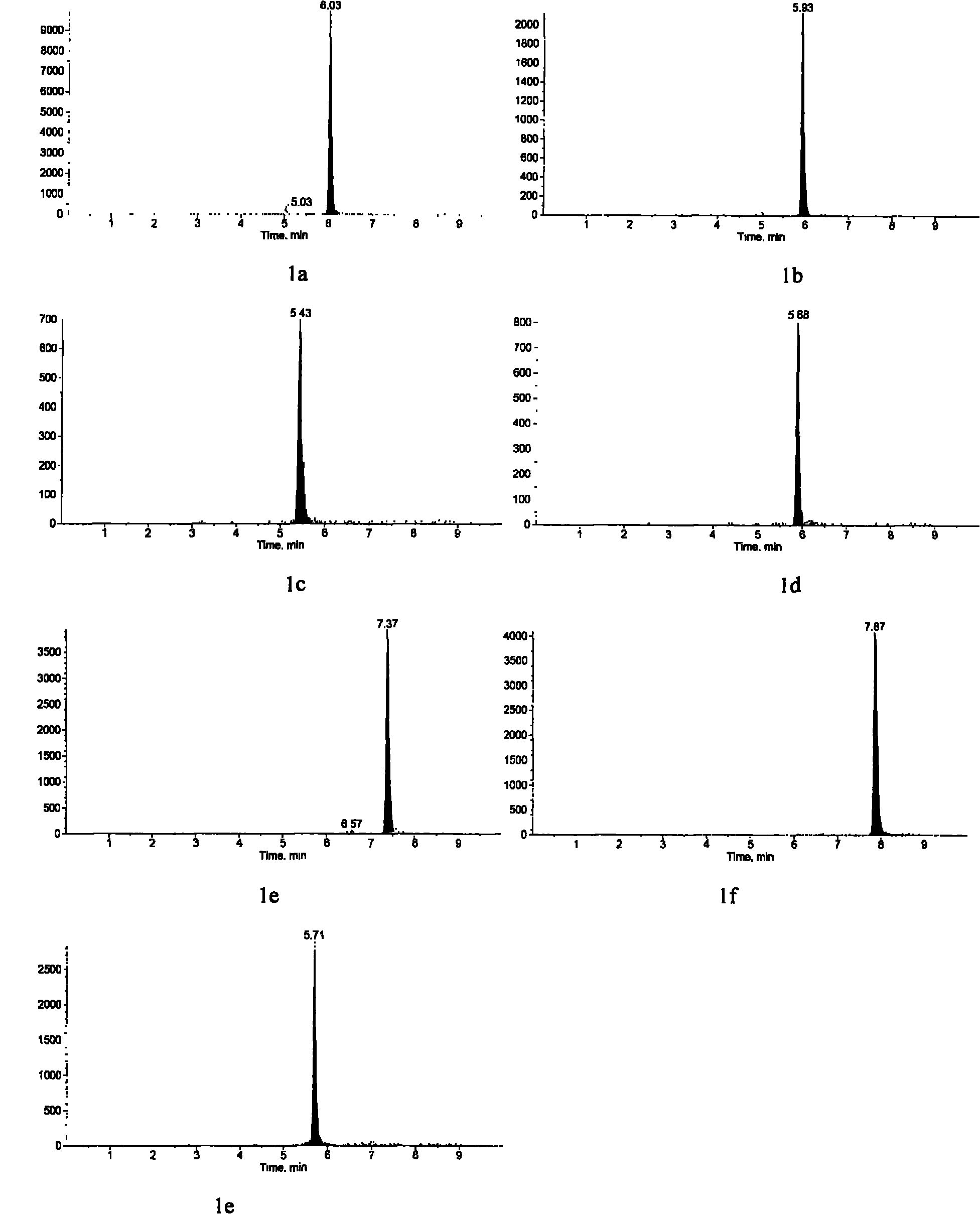
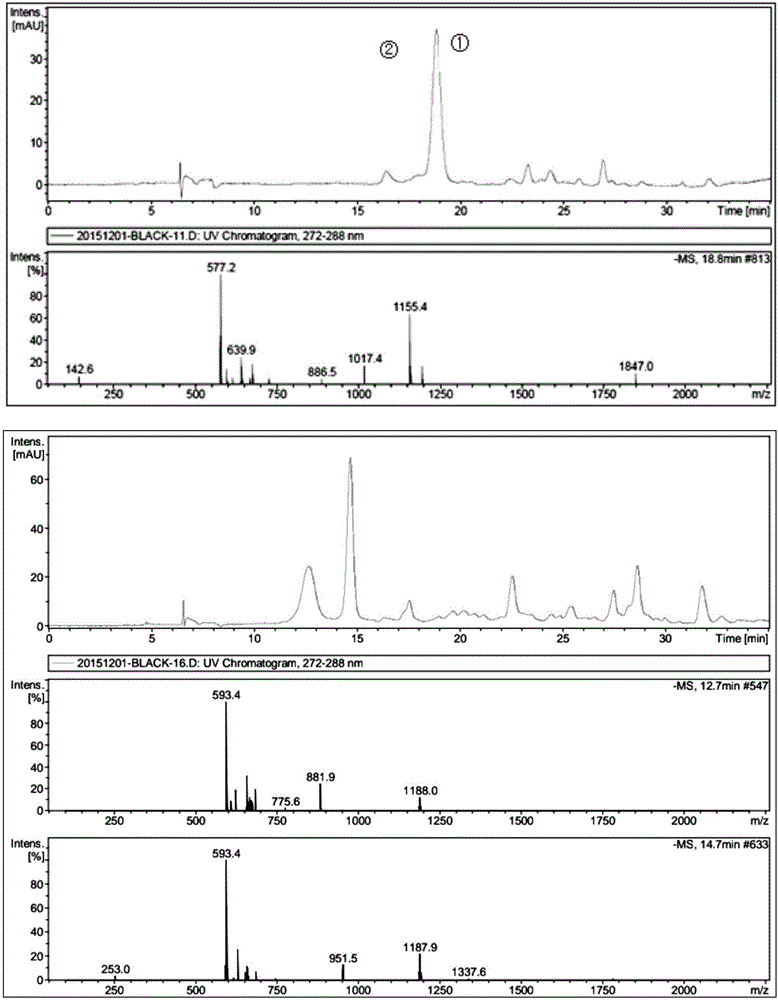
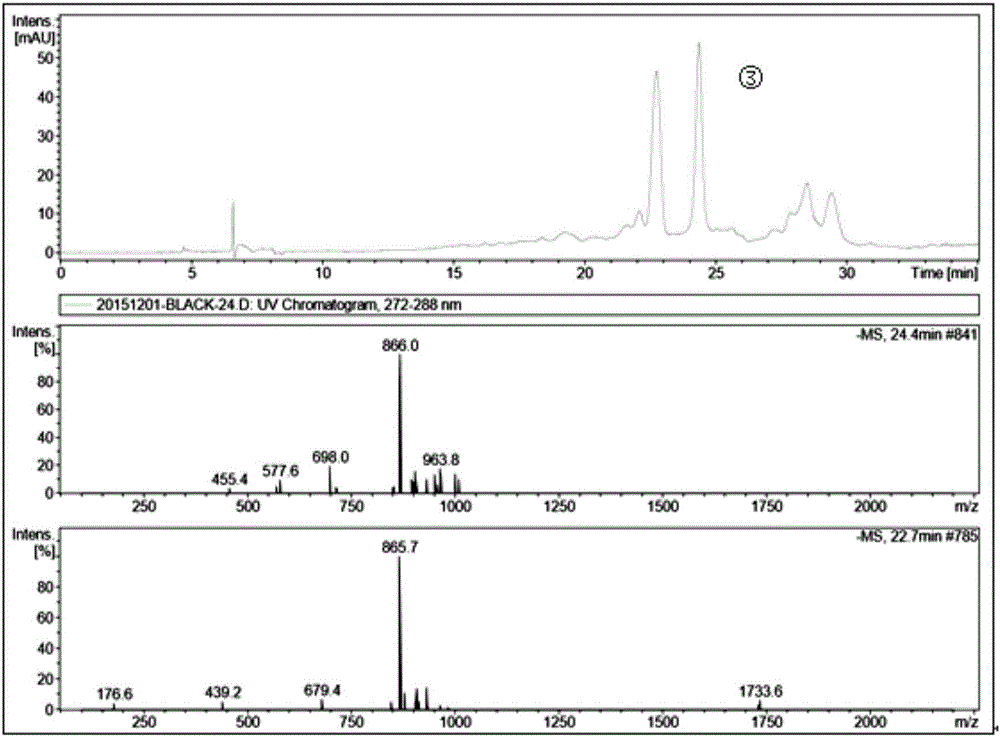
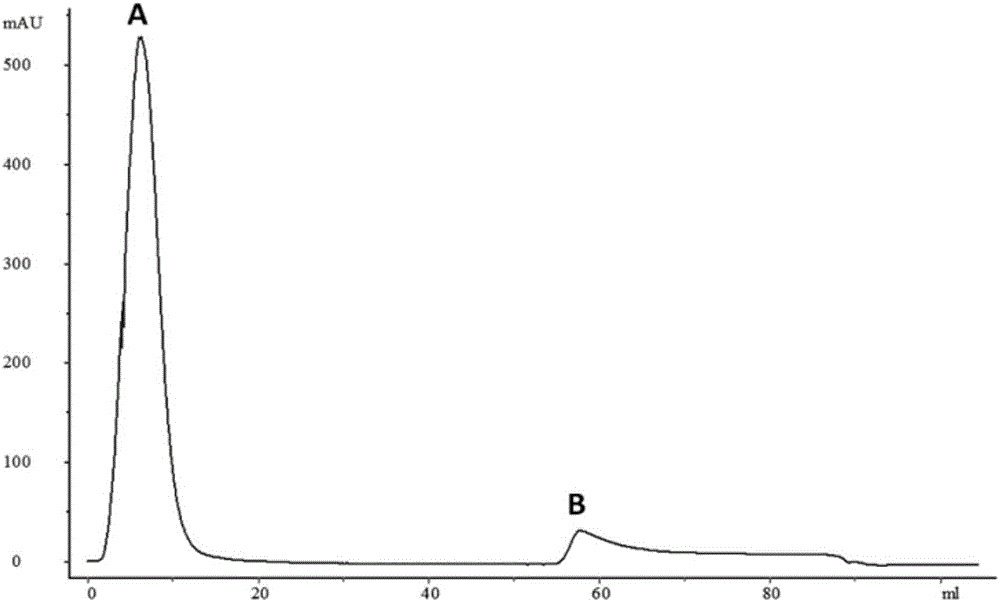
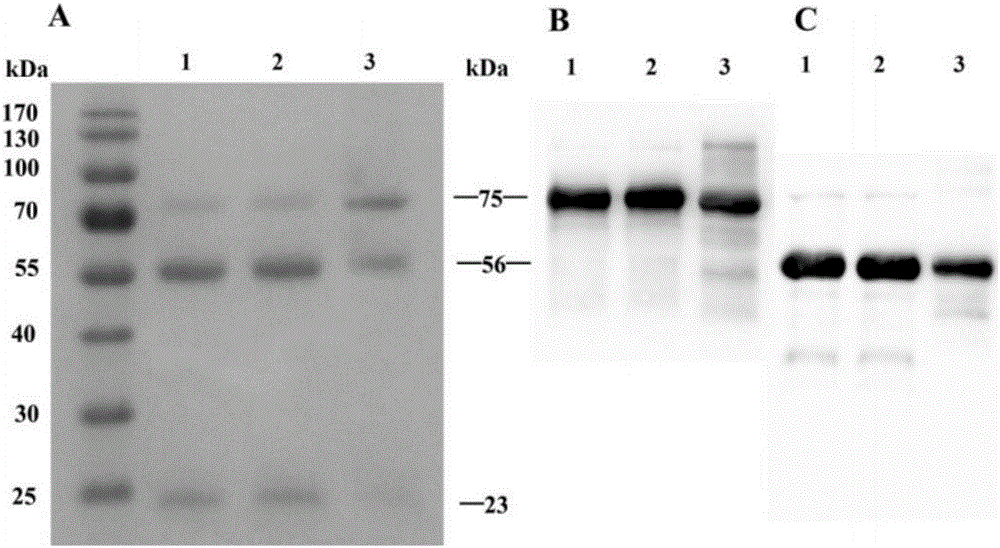
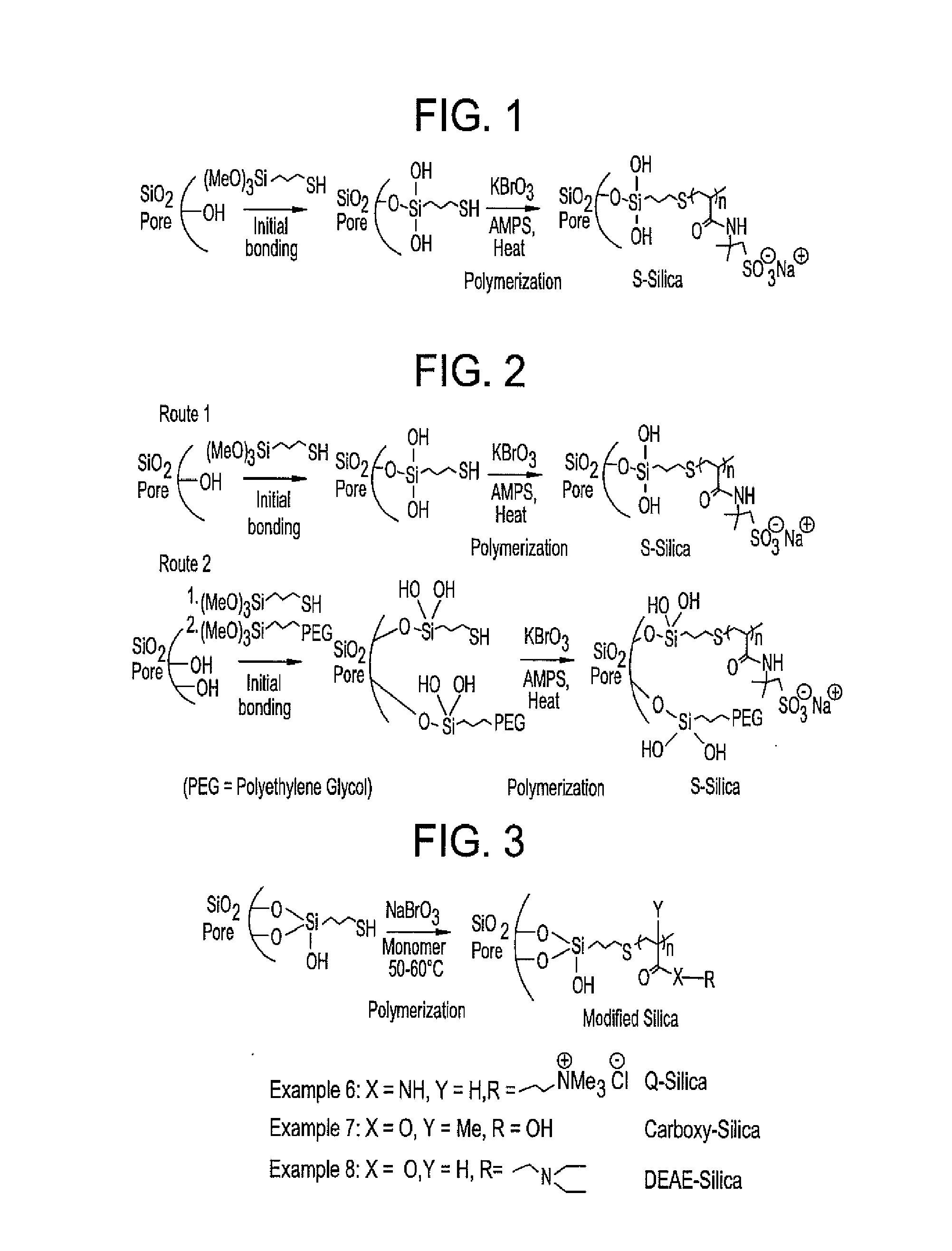
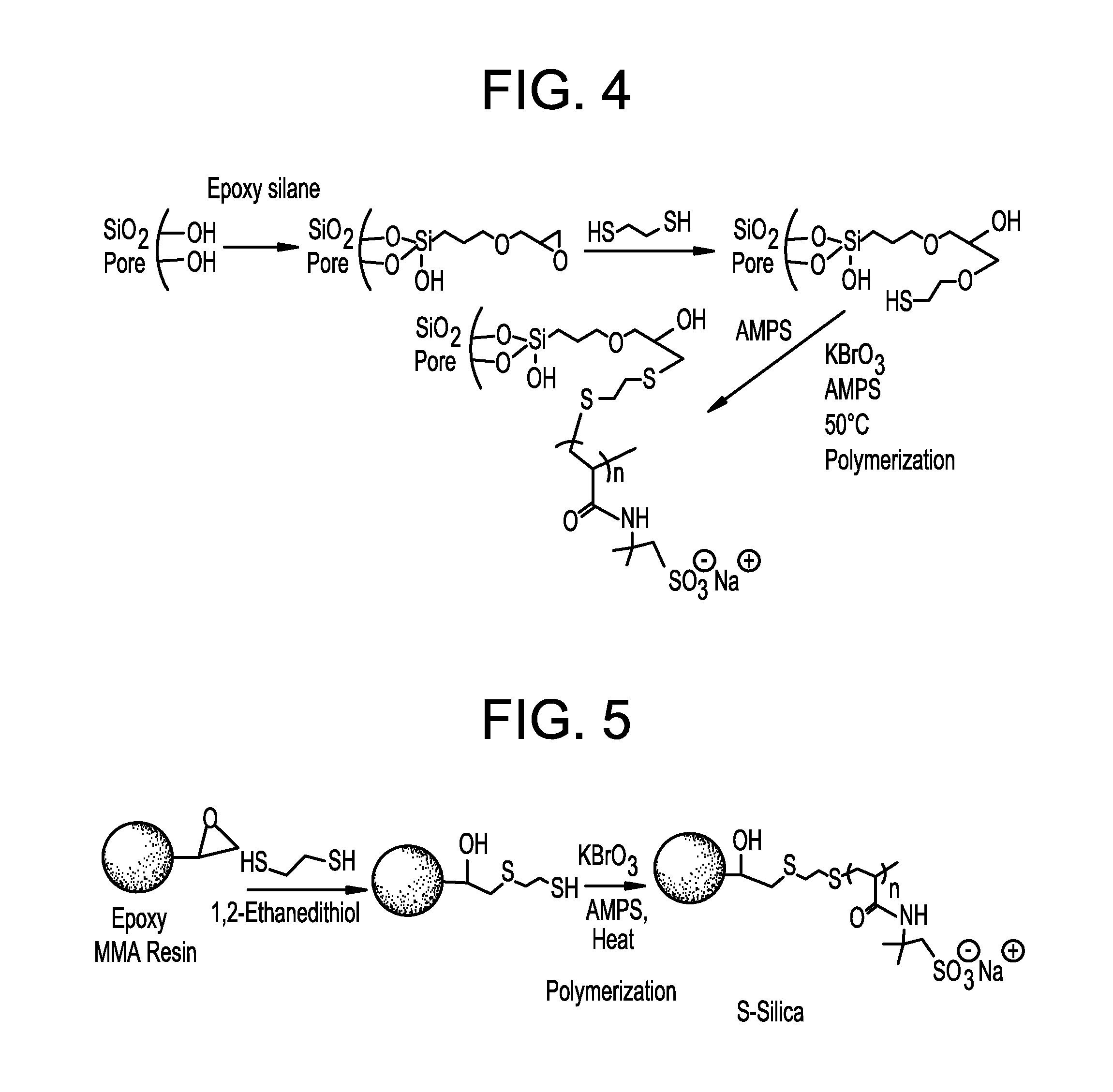
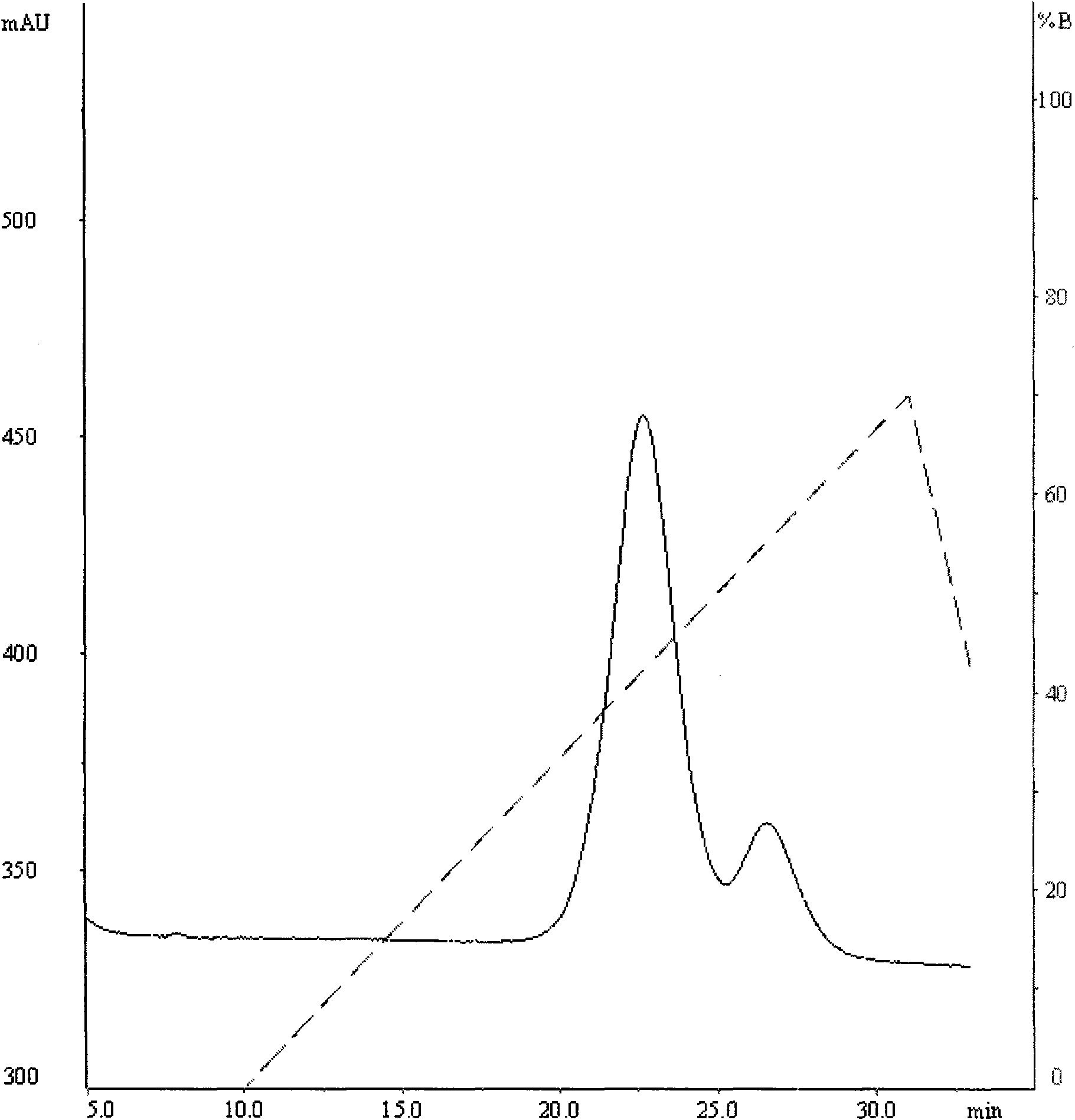
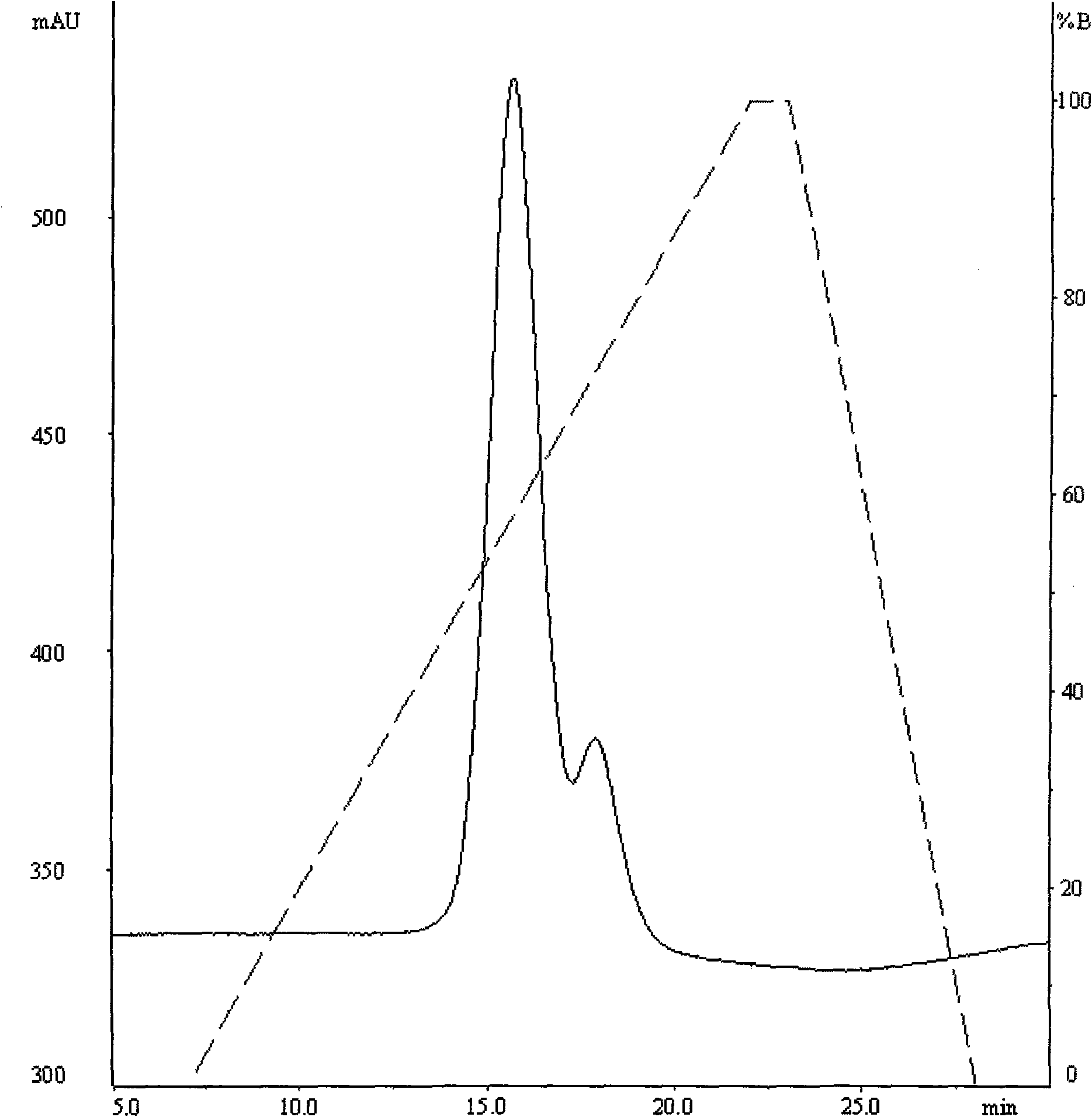
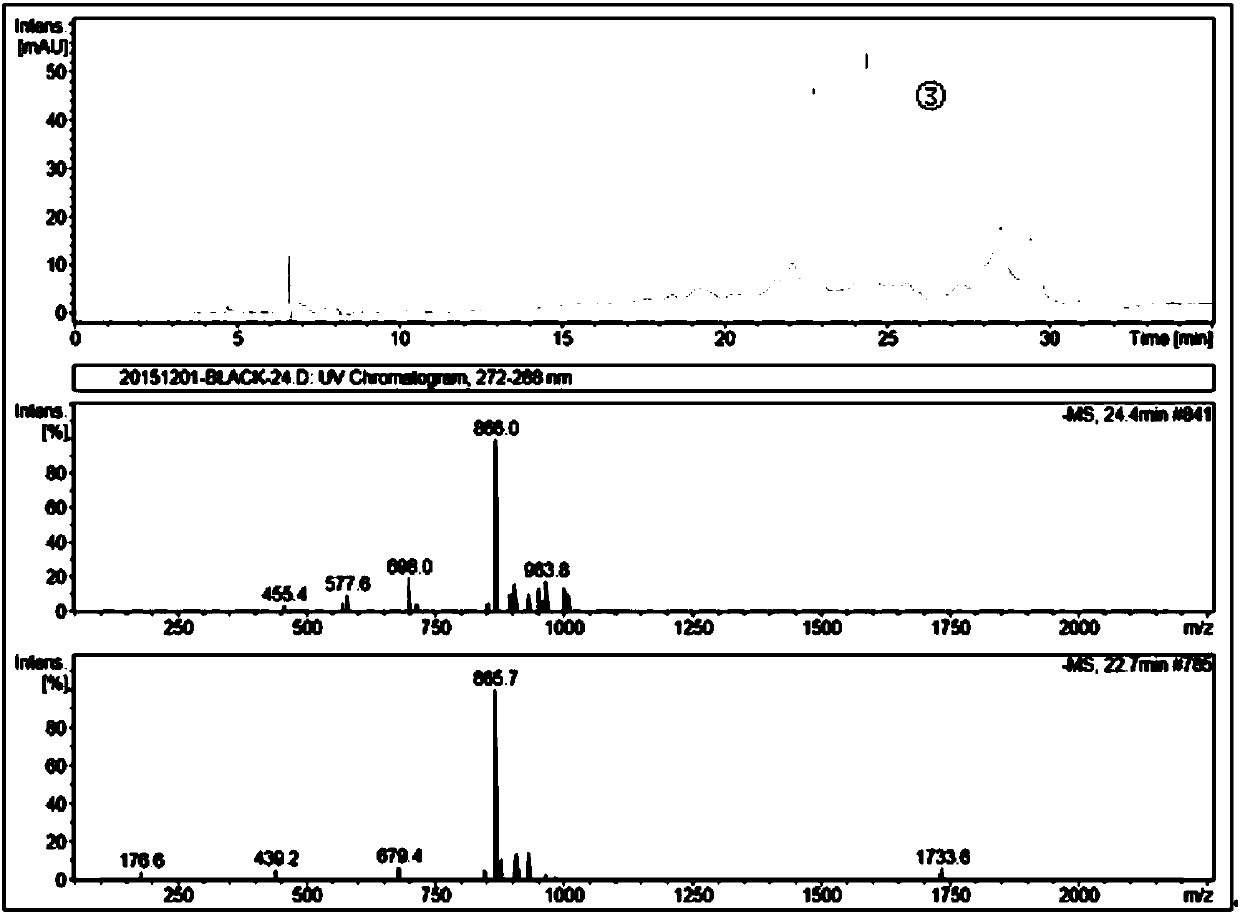
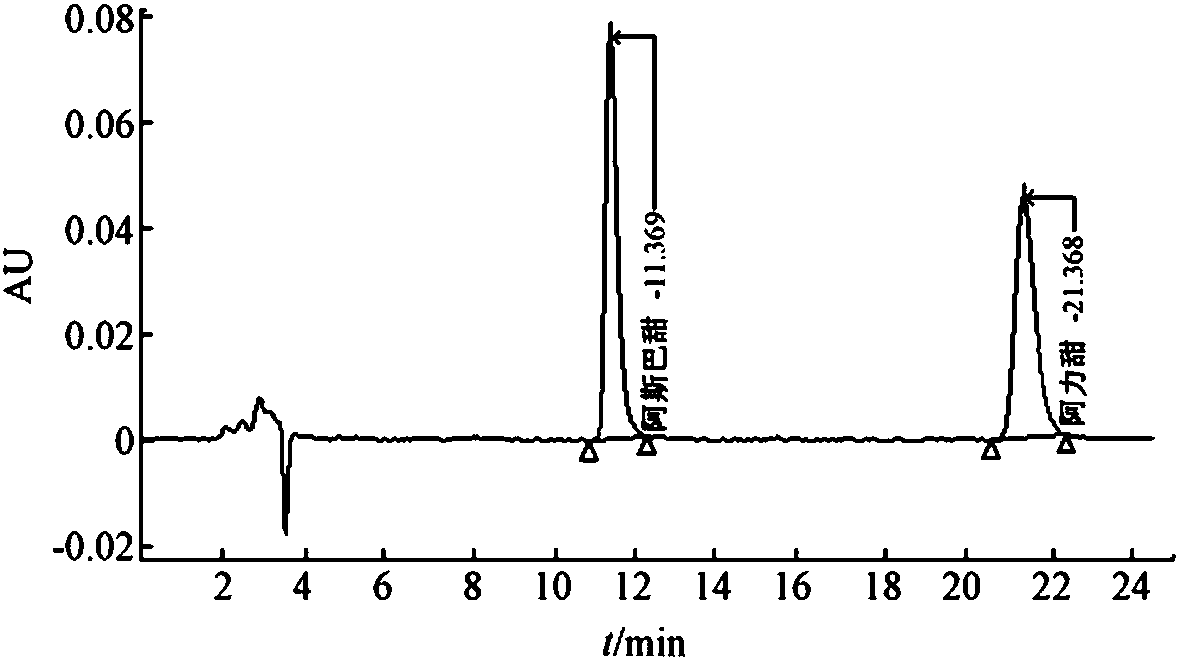
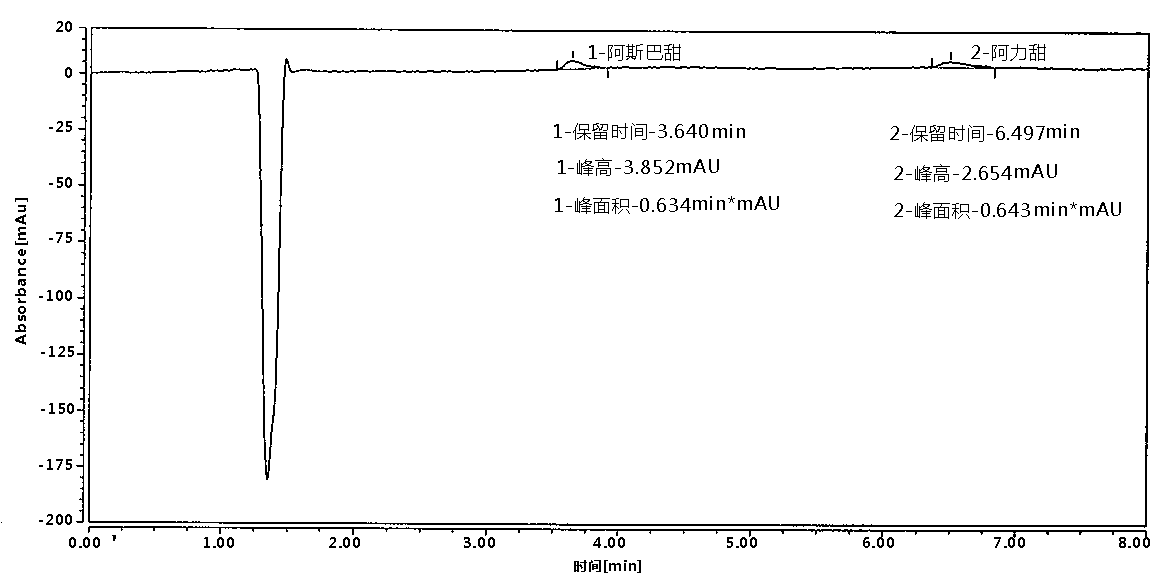
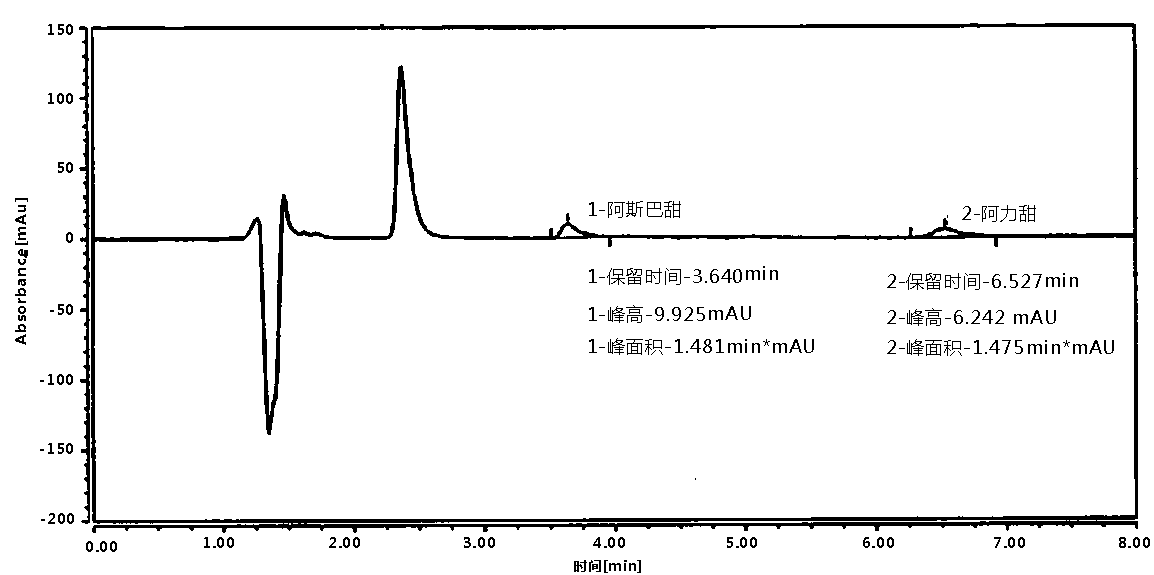
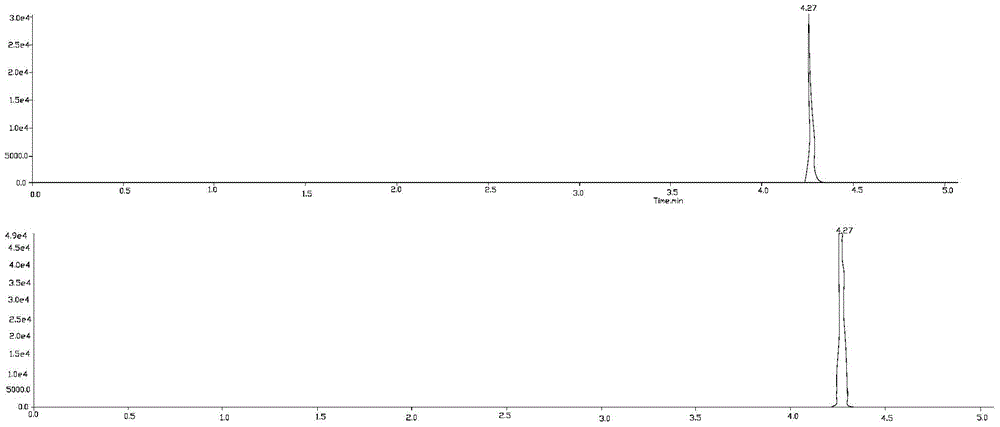
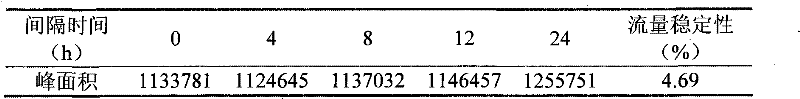
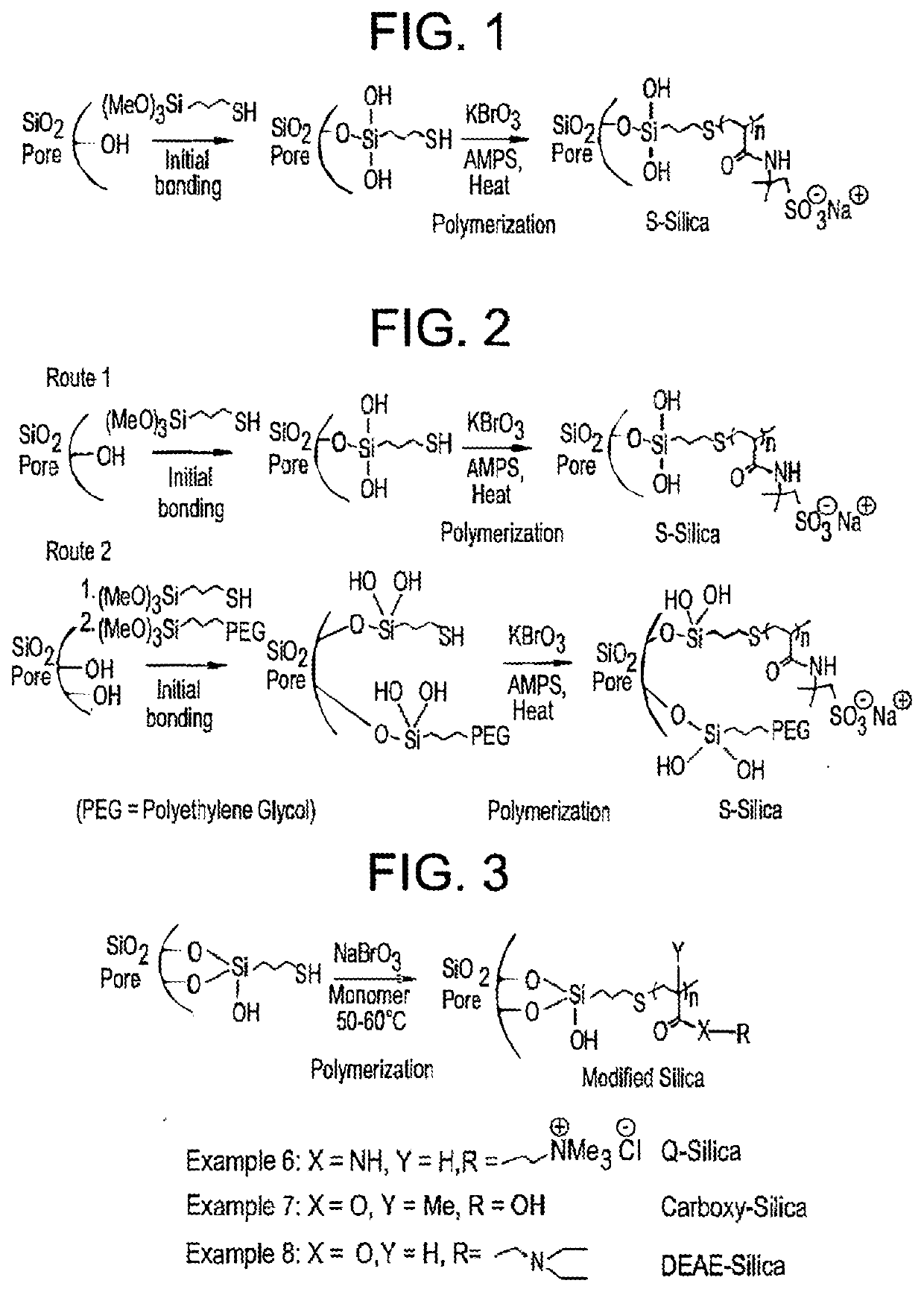
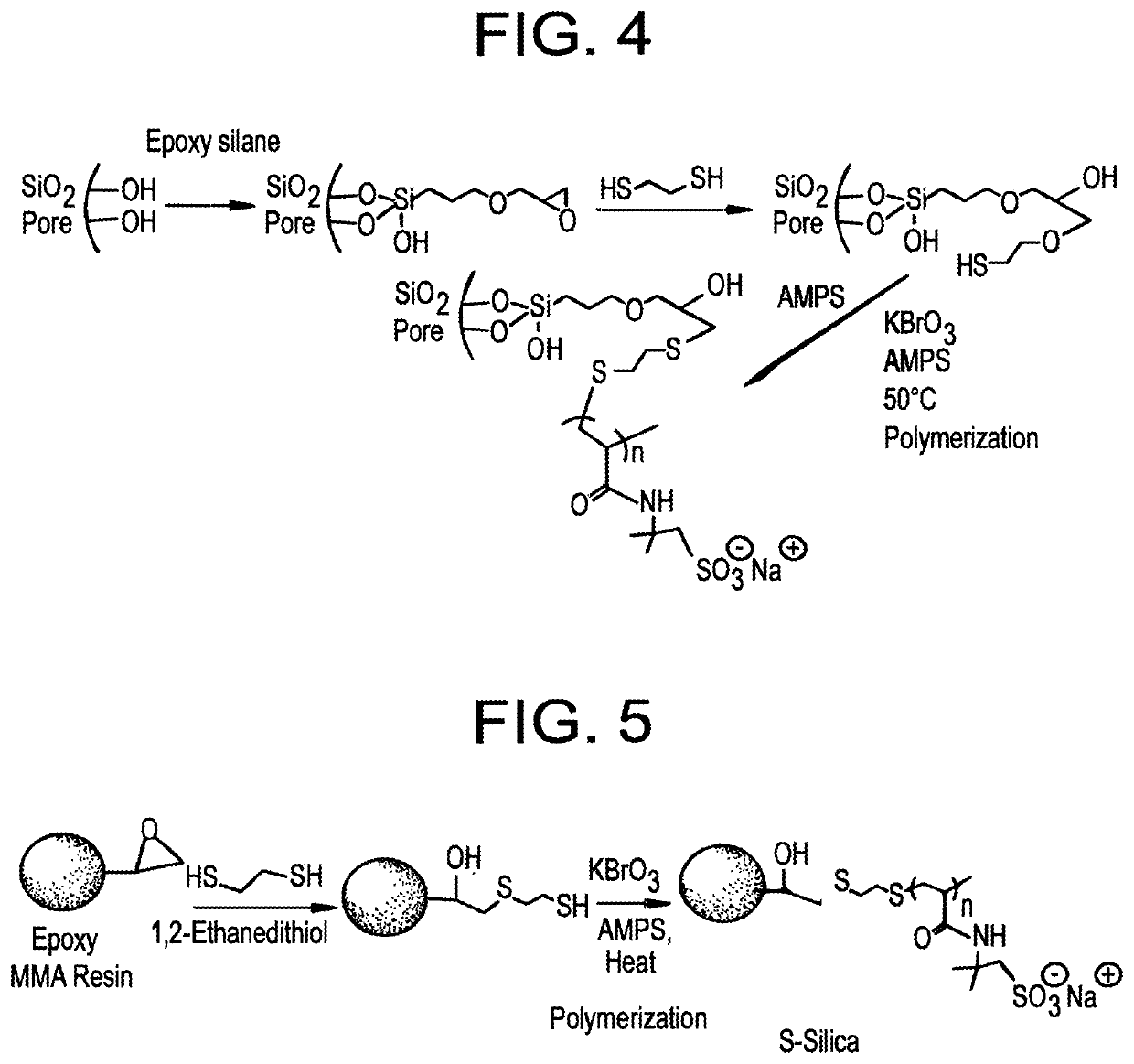
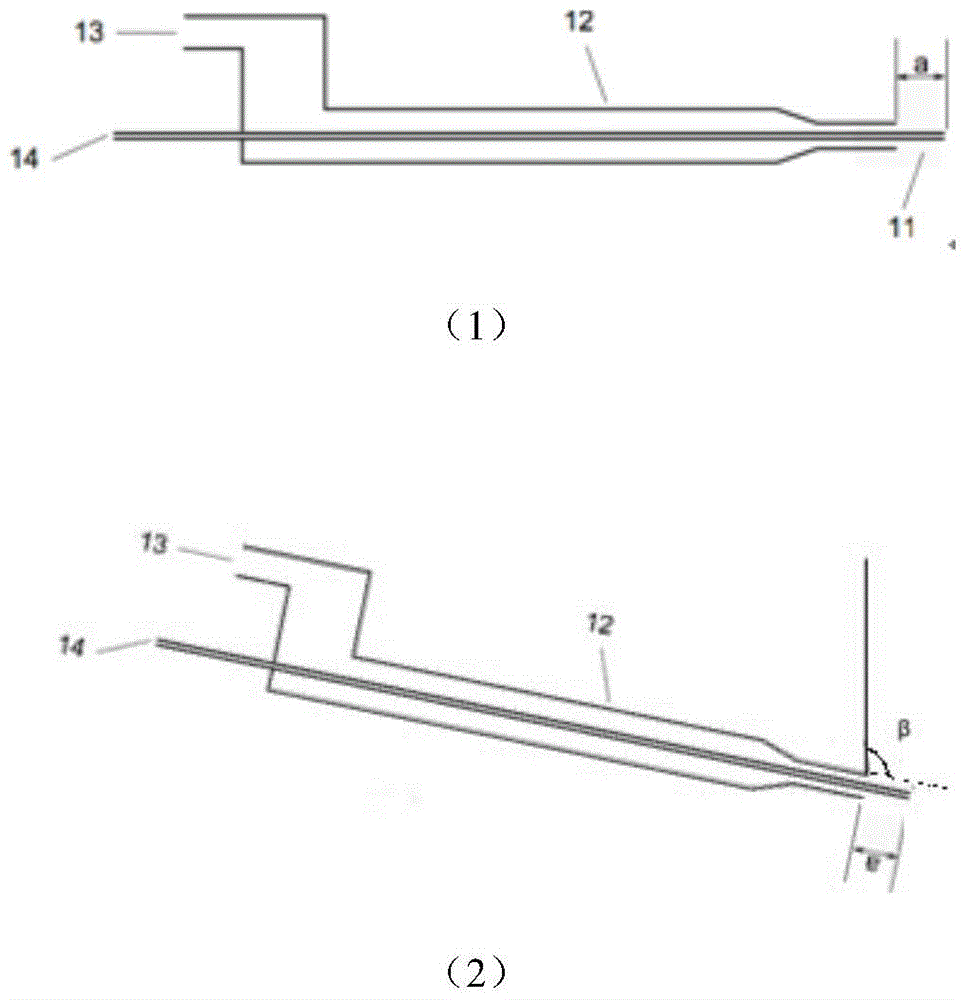
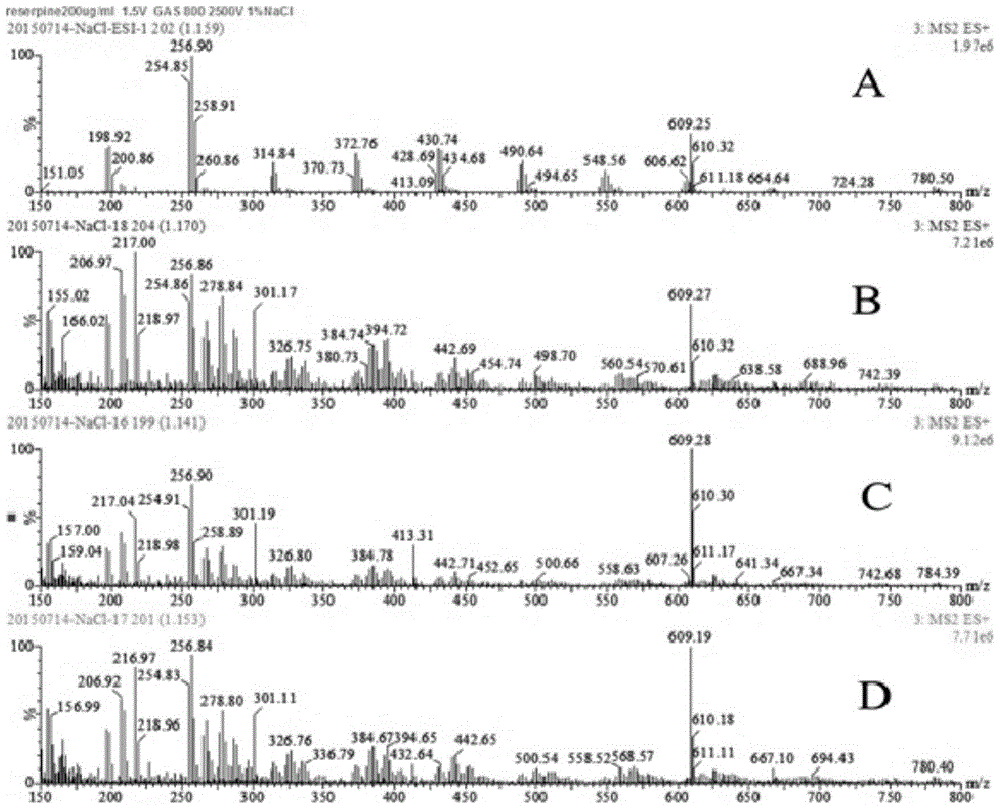
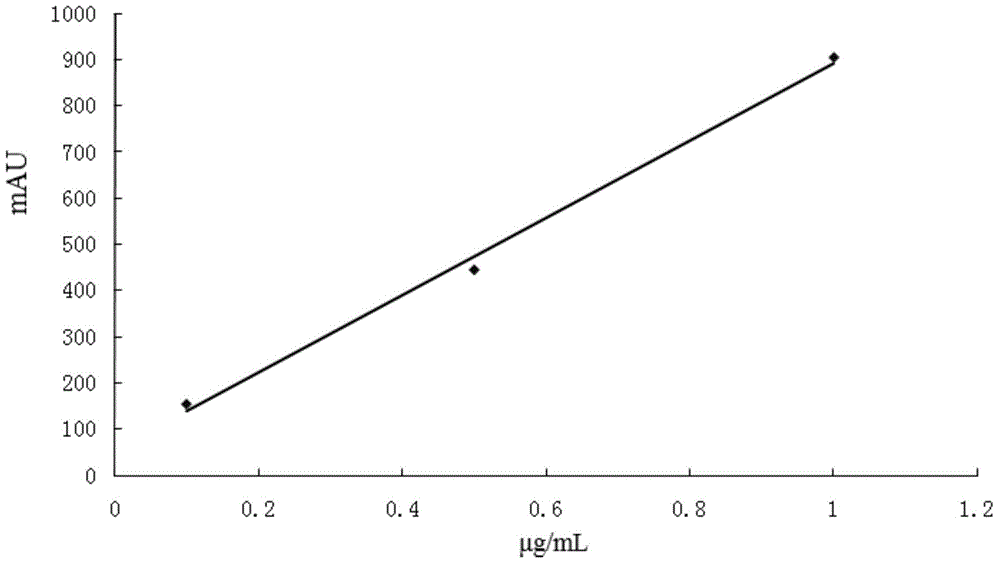
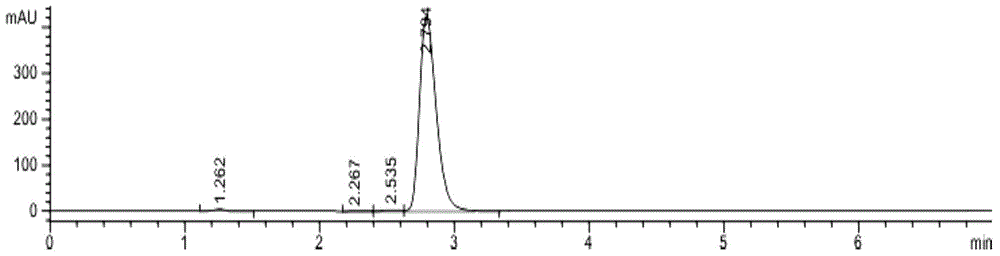
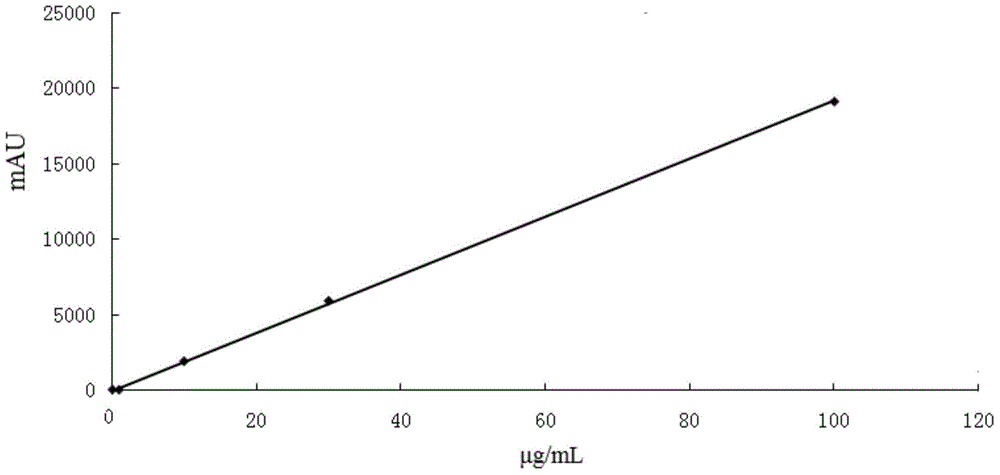
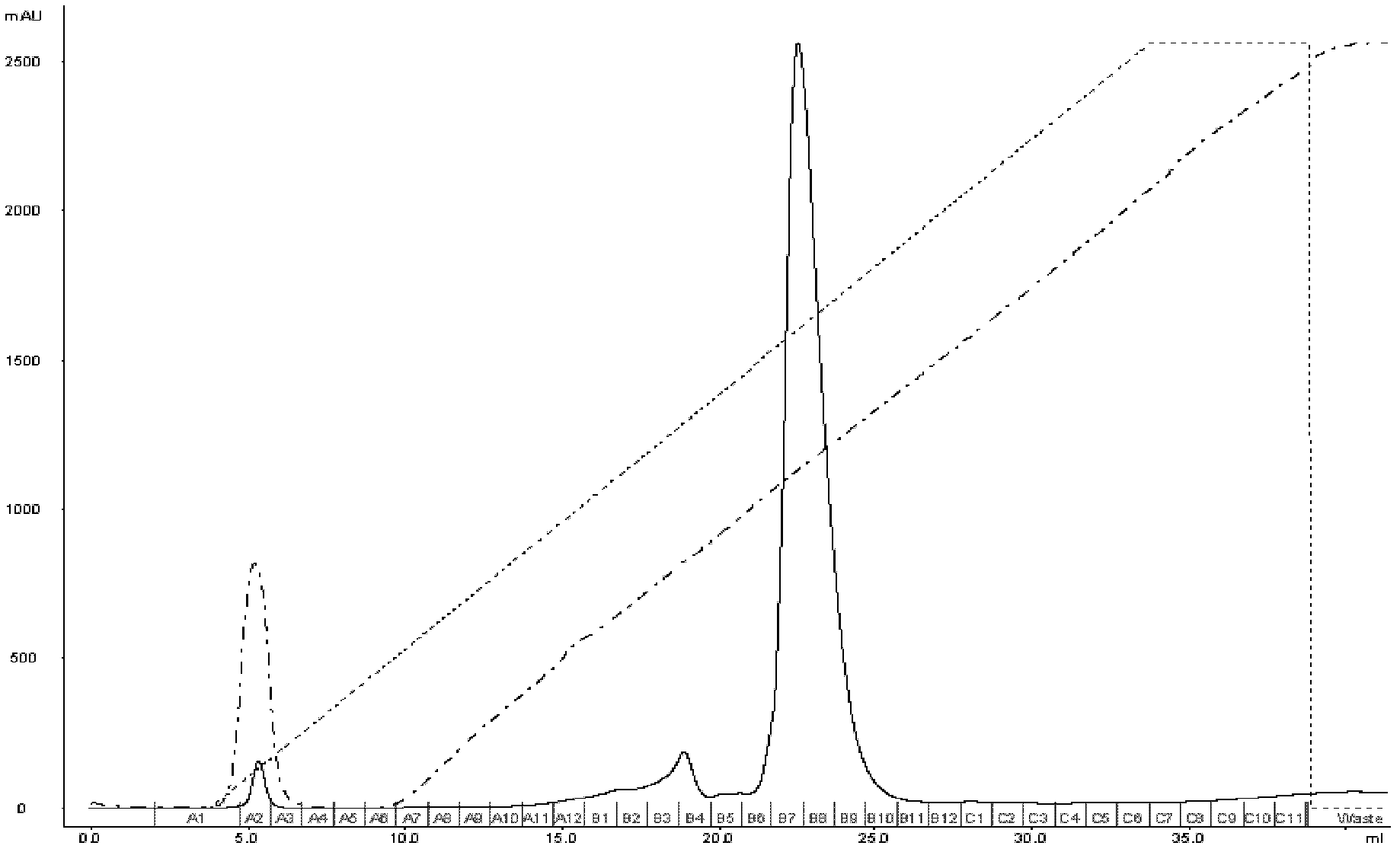
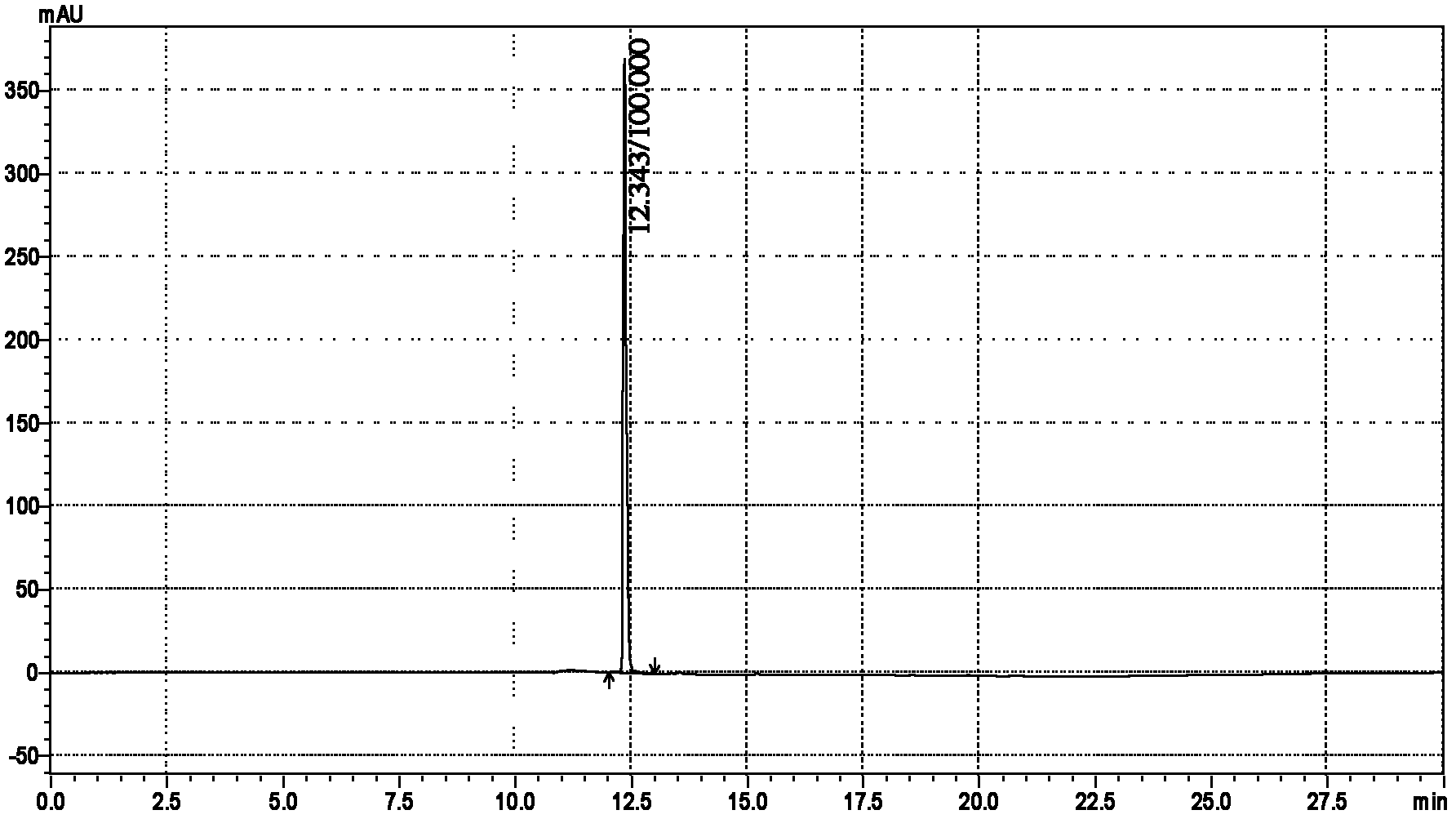
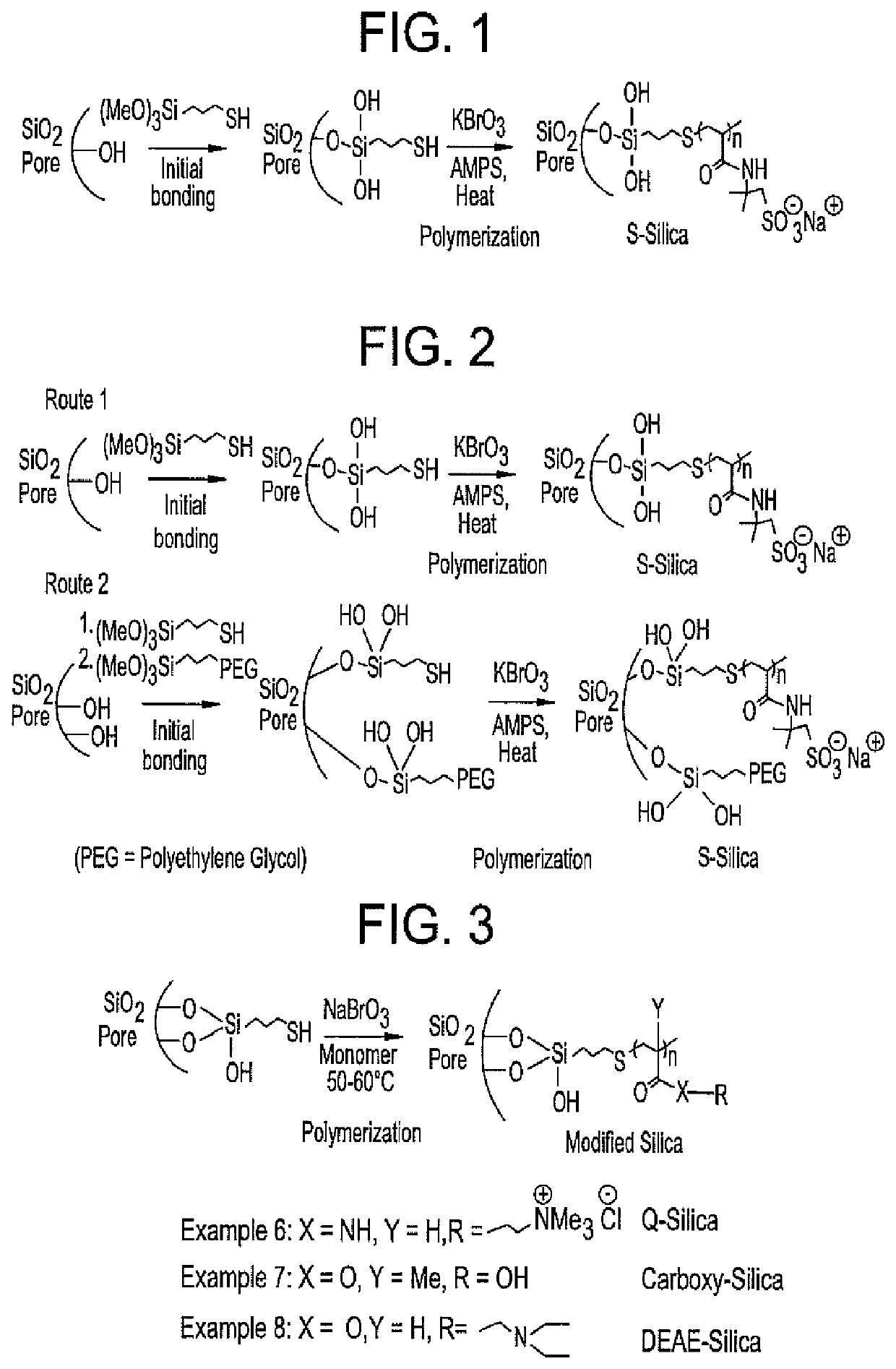
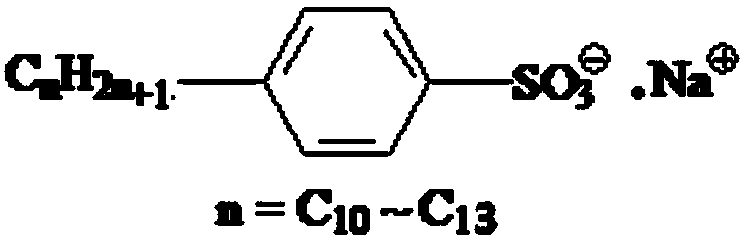Patents
Literature
34 results about "Fast protein liquid chromatography" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fast protein liquid chromatography (FPLC), is a form of liquid chromatography that is often used to analyze or purify mixtures of proteins. As in other forms of chromatography, separation is possible because the different components of a mixture have different affinities for two materials, a moving fluid (the "mobile phase") and a porous solid (the stationary phase). In FPLC the mobile phase is an aqueous solution, or "buffer". The buffer flow rate is controlled by a positive-displacement pump and is normally kept constant, while the composition of the buffer can be varied by drawing fluids in different proportions from two or more external reservoirs. The stationary phase is a resin composed of beads, usually of cross-linked agarose, packed into a cylindrical glass or plastic column. FPLC resins are available in a wide range of bead sizes and surface ligands depending on the application.
Method for detecting 25(hydroxyl)vitamin D by using high-pass liquid chromatography-tandem mass spectrometry
ActiveCN103308621AThe pre-processing process is simpleStrong specificityComponent separationRetention timeMass analyzer
The embodiment of the invention provides a method for detecting 25(hydroxyl)vitamin D by using a high-pass liquid chromatography-tandem mass spectrometry. The method comprises the following steps of: adding an acetonitrile solution containing an internal standard substance of the 25(hydroxyl)vitamin D into a human serum sample to carry out protein precipitation; sufficiently and uniformly mixing the solution, and then, adding an n-hexane extracting solvent; sufficiently and uniformly mixing the solution, then centrifuging the solution, movably taking a supernatant and drying the supernatant, and adding a complex solution to obtain a sample to be detected; detecting the sample to be detected by using a high-pass liquid chromatography-tandem quadrupole mass spectrometer; and quantifying according to the relative retention time of 25(hydroxyl)vitamin D2 and / or 25(hydroxyl)vitamin D3 and the detected abundance ratio of quantitative ion pairs by using an internal standard curve method. According to the embodiment of the invention, the method has the advantages of simplicity in pretreatment, strong specificity and matrix interference resistance, short detection time, high pass, high detection precision and low cost.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT
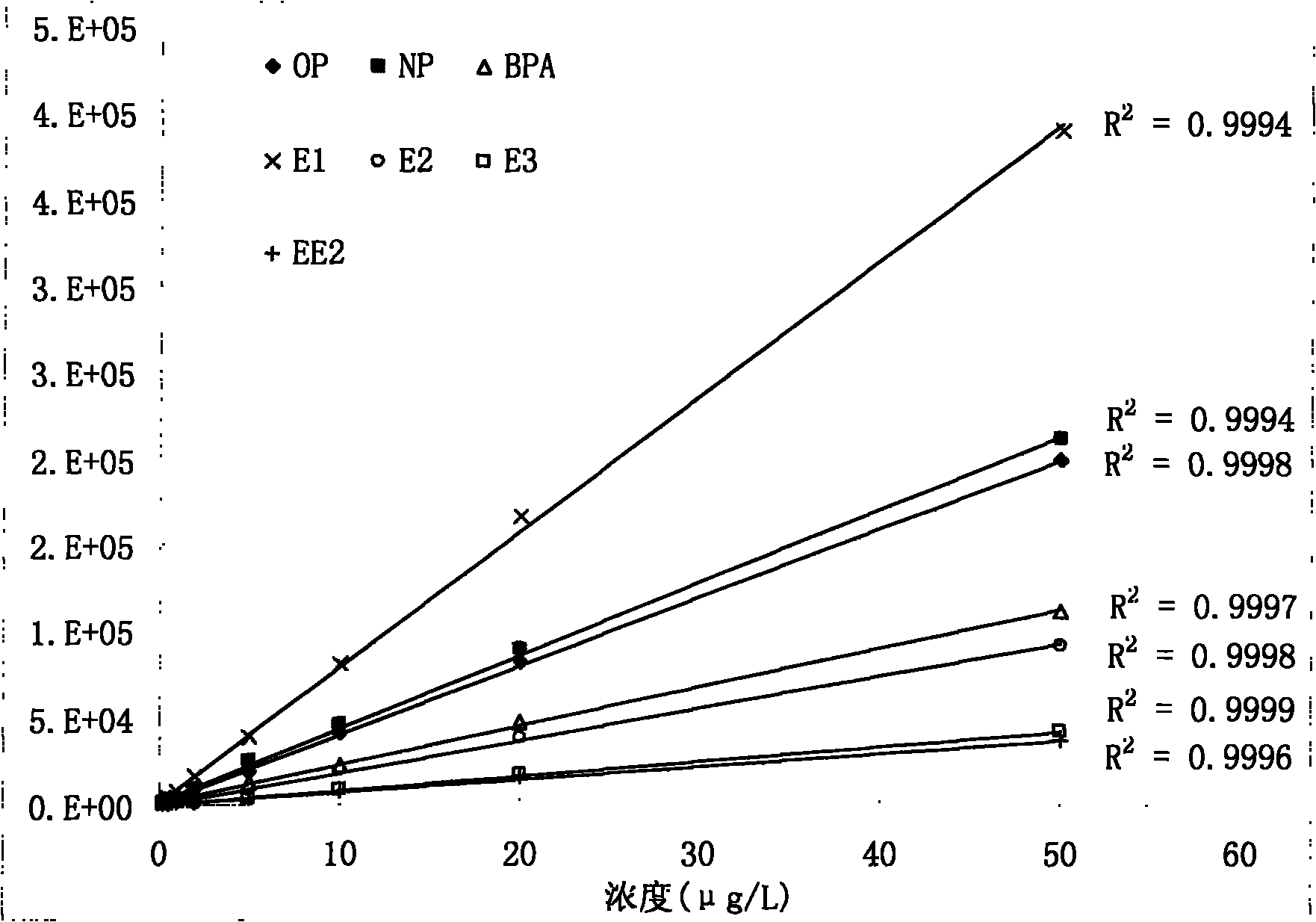
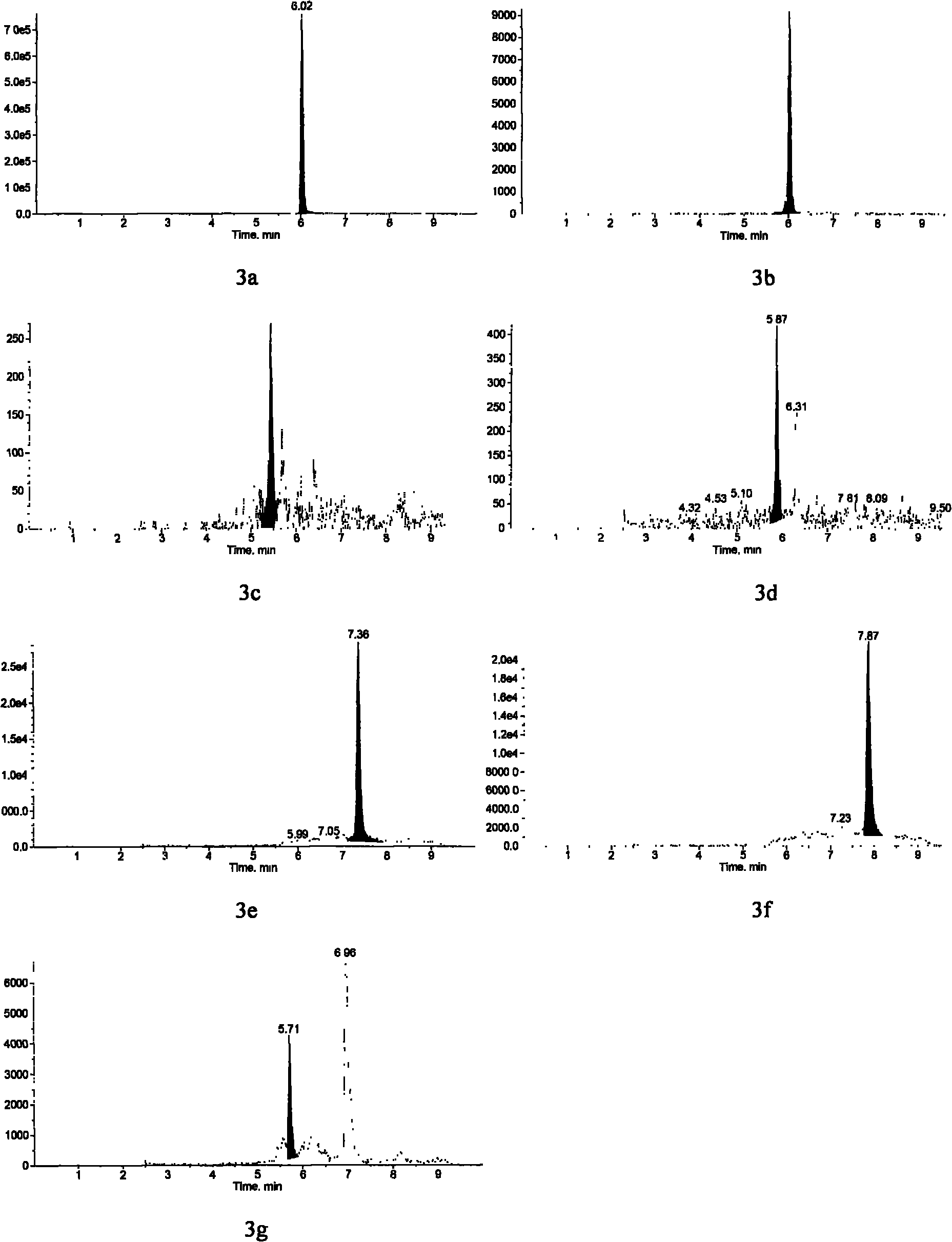
Method for detecting estrogen, nonyl phenol, octyl phenol and bisphenol A in water body sediment together
InactiveCN102183606AEasy to analyzeFast extractionComponent separationAutosamplerLiquid chromatography mass spectroscopy
The invention discloses a method for detecting estrogen, nonyl phenol, octyl phenol and bisphenol A in water body sediment based on an accelerated solvent extraction (ASE), liquid-liquid extraction (LLE) and liquid chromatography / tandem mass spectrum (LC / MS / MS) technology. The method comprises the following steps of: performing accelerated solvent extraction by using acetone as an extracting agent to extract the estrogen, the nonyl phenol, the octyl phenol and the bisphenol A in the water body sediment; dissolving residues generated after extract is blown by nitrogen by using 1mol / L sodium hydroxide solution, performing centrifugal collection on the supernate, and performing liquid-liquid extraction by using ethyl acetate under the condition that the pH is equal to 2; and performing concentration detection by using a 3200QTRAP type liquid chromatography / tandem mass spectrometer and an Agilent 1100 high performance liquid chromatography system of American Applied Biosystem Company and an automatic sample injector of American Agilent Company. The recovery rate is 59 to 94 percent, and the detection limit is 0.04ng / g.
Owner:BEIJING NORMAL UNIVERSITY
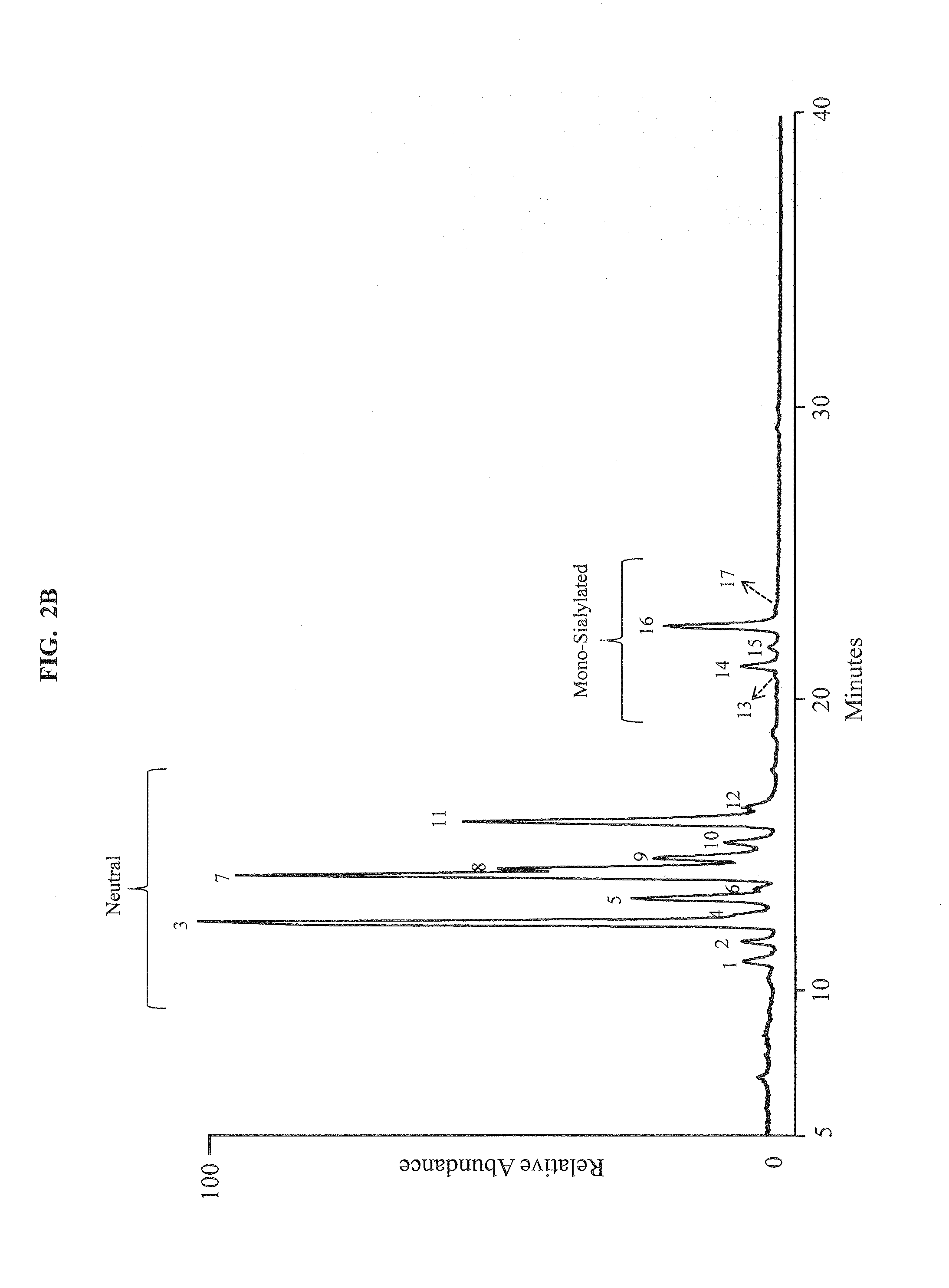
Separation of glycans by mixed-mode liquid chromatography
ActiveUS20140178912A1Highly versatileDesirable selectivitySilicon organic compoundsComponent separationChromatographic separationBinding site
An exemplary multimodal chromatographic medium of the invention includes one or more strong anion exchange, weak anion exchange, strong cation exchange and / or weak cation exchange binding sites in combination with one or more reverse phase and / or hydrophilic interaction chromatography binding site. In an exemplary embodiment, the sites interact with one or more glycans in a mixture of glycans in a manner that allows separation of glycans in the mixture and analysis of the glycan mixture. The media are incorporated into devices and systems for chromatographic analysis. Also provided are methods of using the multimodal media of the invention to analyze glycans.
Owner:DIONEX CORP
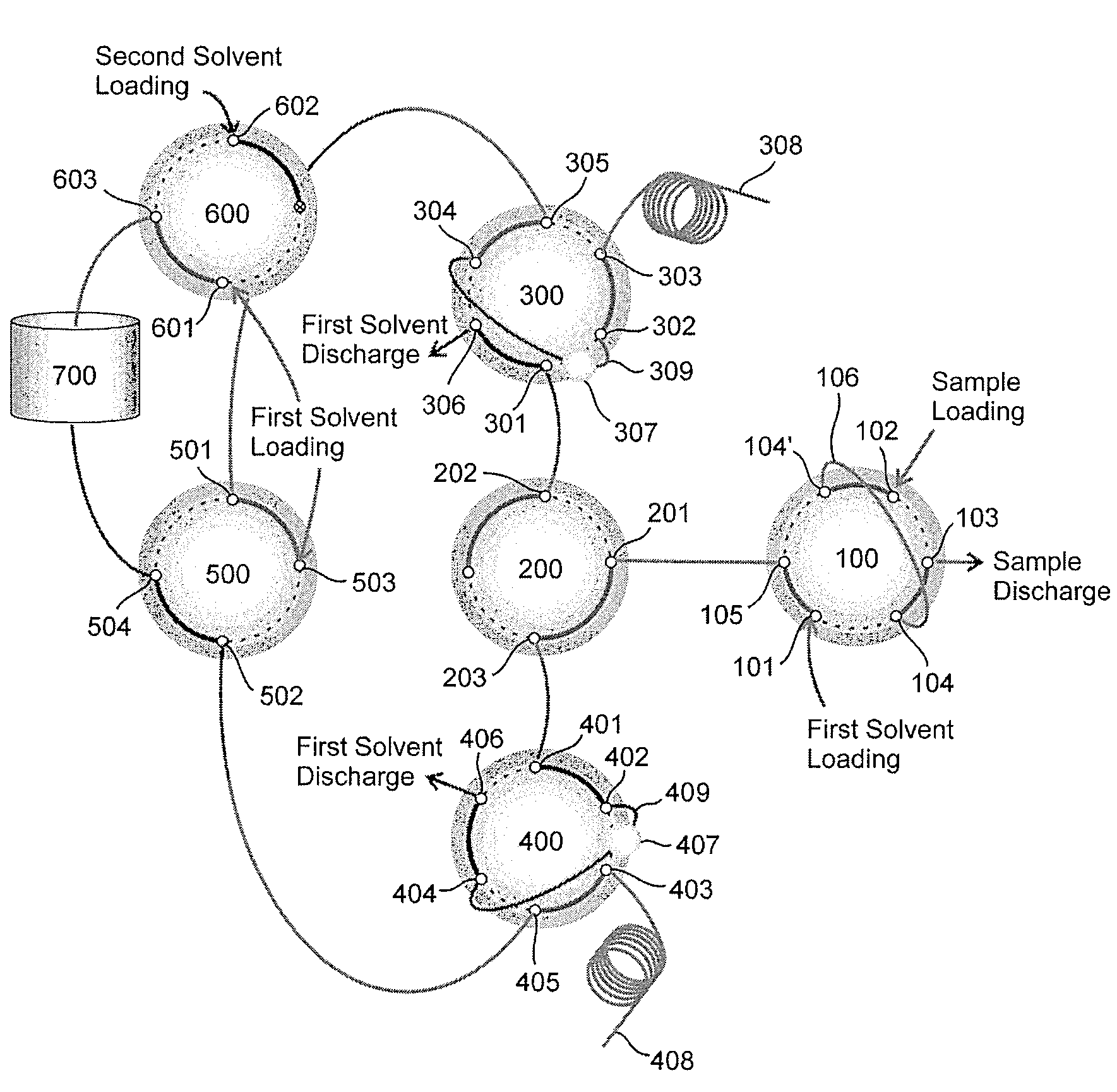
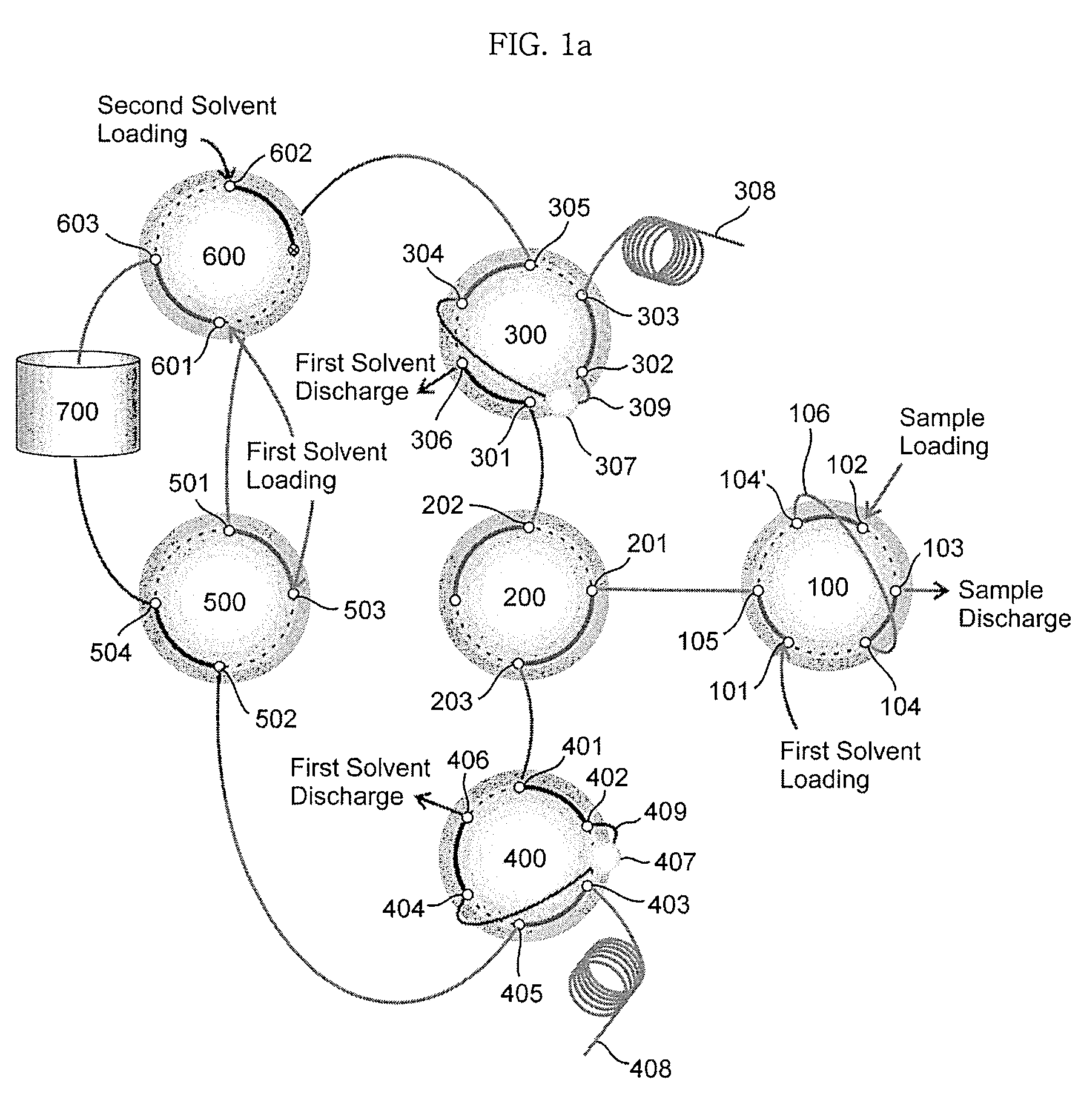
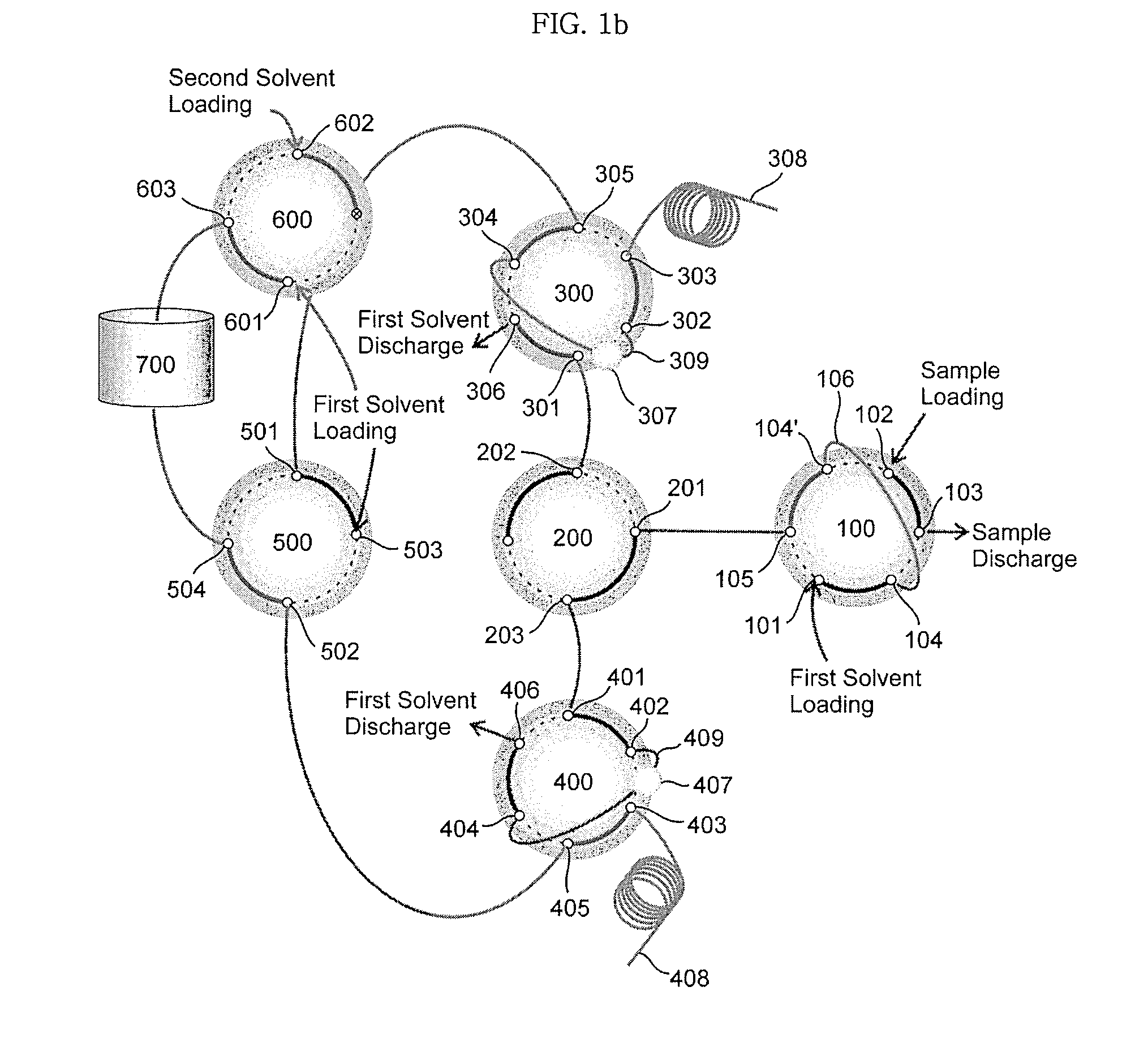
Ultrahigh-pressure dual on-line solid phase extraction/capillary reverse-phase liquid chromatography system
An ultrahigh-pressure dual on-line solid phase extraction / capillary reverse-phase liquid chromatography (DO-SPE / cRPLC) system is provided. The system comprises: a sample loading valve into which a first solvent and a sample to be analyzed are loaded; a first column valve in flow communication with a first solid-phase extraction column and a first reverse-phase liquid chromatography column; a second column valve in flow communication with a second solid-phase extraction column and a second reverse-phase liquid chromatography column; a column-switching valve for determining whether the sample is transferred to either the first column valve or the second column valve; a solvent selection valve in flow communication with the first and second column valves to supply the first solvent or a mixed solvent of the first solvent and a second solvent to the first and second column valves; a second solvent loading valve, in flow communication with a solvent mixer, into which the first and second solvents are loaded; a supply pump for loading the second solvent into the second solvent loading valve; and a supply pump for loading the first solvent into the sample loading valve, the second solvent loading valve and the solvent selection valve. The system requires minimal time (i.e. dead time) for column equilibration between successive experiments to shorten the total time required for the experiments by a factor of about two. In addition, the system enables rapid sample injection, on-line sample desalting and sample enrichment. Furthermore, the system is highly reproducible in terms of liquid chromatography (LC) retention time and can be operated at a pressure as high as 10,000 psi.
Owner:HANBAT NAT UNIV IND ACADEMIC COOPERATION FOUND
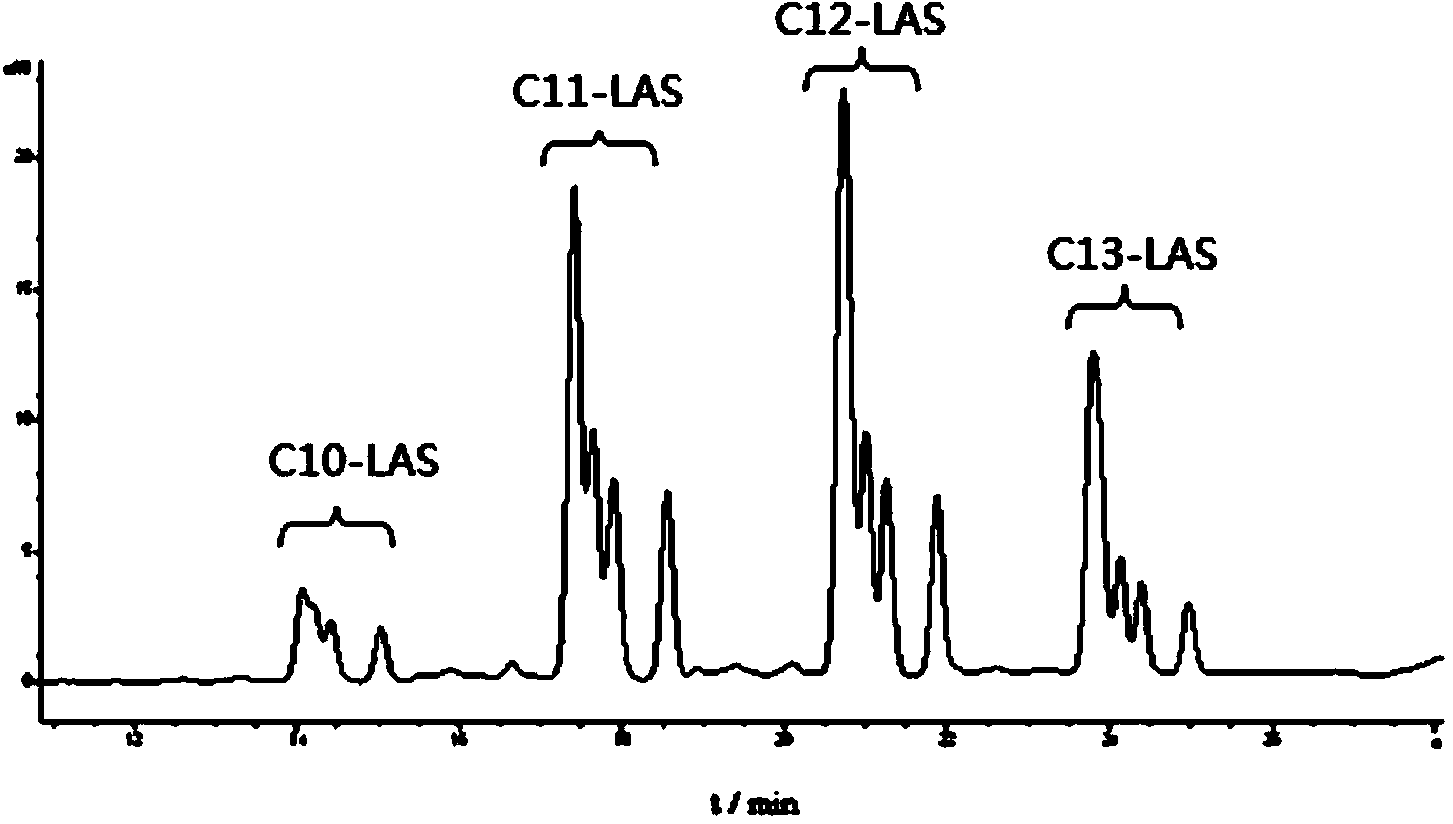
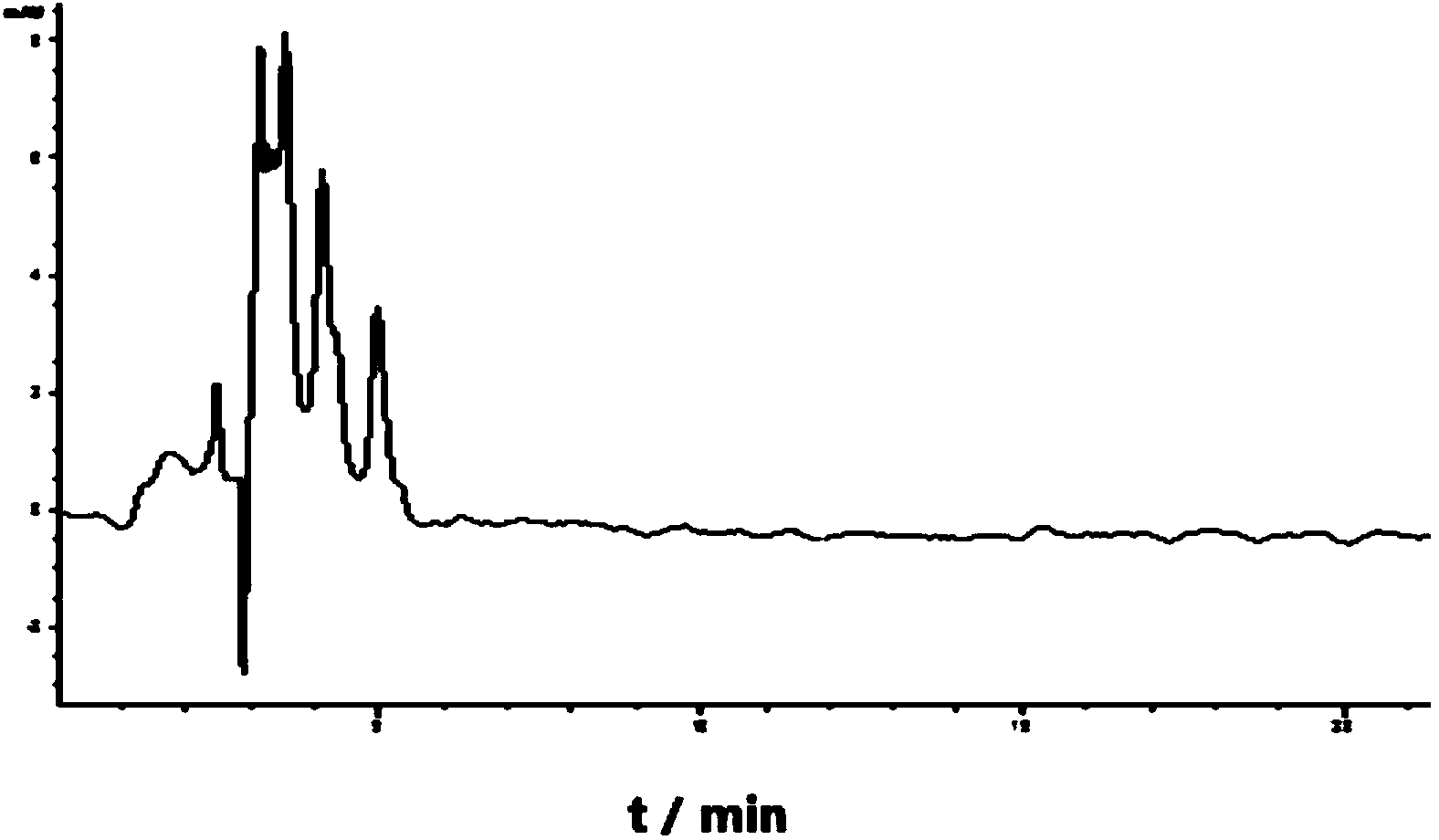
Method for determining linear alkylbenzene sulfonate (LAS) in textile dyeing and finishing auxiliaries by ion-pair liquid chromatography
ActiveCN103728398ALess spectral interferenceStrong specificityComponent separationChromatographic separationAnti jamming
The invention discloses a method for determining linear alkylbenzene sulfonate (LAS) in textile dyeing and finishing auxiliaries by ion-pair liquid chromatography, belonging to the analysis and detection fields of LAS residue in the textile dyeing and finishing auxiliaries. The method comprises the steps of firstly, sample solution preparation, namely, dissolving a sample with a strong polar solvent under the assistance of ultrasonic waves; secondly, solid-phase extraction and purification; and thirdly, separating by the ion-pair liquid chromatography and detecting by a fluorescence detector. The LAS is used as a standard, the chromatographic retention times of LAS in a comparative sample solution and a standard solution are compared, the special fingerprint spectrum is qualitatively analyzed and the sum of the peak areas of four main components of the LAS is quantified by an external standard method. The method for determining the linear alkylbenzene sulfonate (LAS) in textile dyeing and finishing auxiliaries by the ion-pair liquid chromatography is high in detection sensitivity, strong in anti-jamming capability and accurate in qualitative and quantitative analysis.
Owner:南京海关工业产品检测中心
Preparation method of magnetic molecularly imprinted composite material of chiral rodenticide bromadiolone
The invention discloses a preparation method of a magnetic molecularly imprinted composite material of chiral rodenticide bromadiolone. The preparation method comprises the following steps of: adding magnetic nanometer ferroferric oxide particle modified via oleic acid to a dispersing agent; adding polymerizing monomer, functional monomer and cross-linking agent; ultrasonically dispersing; adding an initiator; heating; agitating to react to obtain single dispersive polymer composite microsphere rich in epoxy groups; washing until pH (Potential Of Hydrogen) reaches 6 to 8; then drying; adding composite reacting liquid of bromadiolone and an amino functional agent; heating; agitating to react; magnetically separate; washing until the pH reaches 6 to 8; adding organic solvent; ultrasonically eluting to remove template molecule bromadiolone until no bromadiolone an be detected in the eluant through superfast efficient liquid chromatography-tandem mass spectrum; and then drying to obtain a target product. The preparation method disclosed by the invention has the characteristics of being simple in preparation technology, controllable in magnet content, and controllable in ratio of functional group and the like; a product is applicable to enriching, purifying, analyzing and detecting of bromadiolone residual in a biological sample; and the preparation method has a wide application prospect.
Owner:NINGBO MUNICIPAL CENT FOR DISEASE CONTROL & PREVENTION
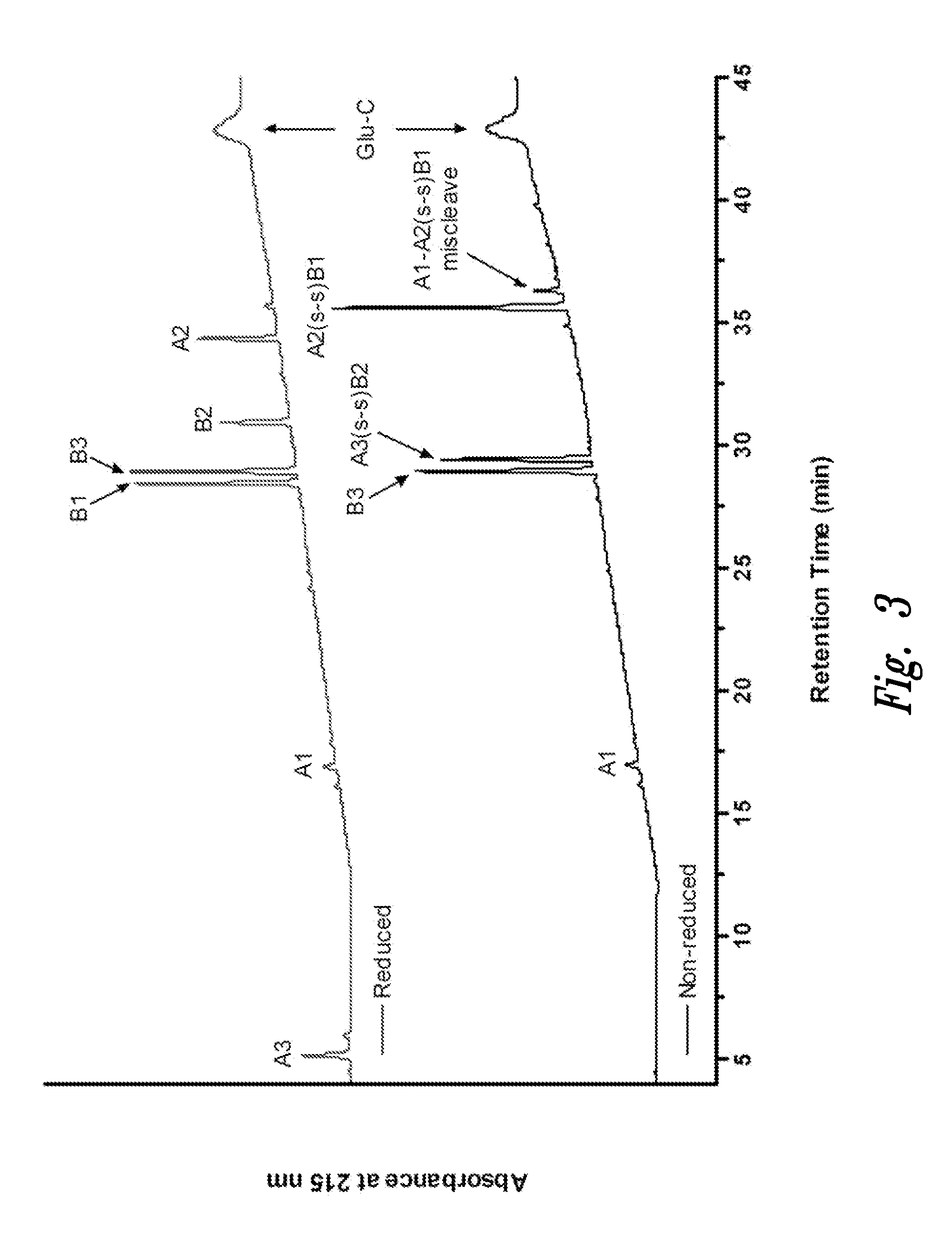
Polypeptide disulfide bond analysis
InactiveUS20110151493A1Improve throughputSimple and rapid and accurate measurement of disulfide bondMicrobiological testing/measurementBiological testingCysteine thiolateCombinatorial chemistry
The present invention relates in part to methods for determining bonding patterns in disulfide-linked peptides containing closely-spaced cysteine residues. Through N-terminal sequencing chemistry coupled with facile liquid chromatography and mass spectrometric analysis of the cleavage products, one can assign connectivity to specific cysteine pairs. A particular advantage of this method is maintenance of disulfide integrity during the process.
Owner:AMGEN INC
Separation and purification method of c-di-GMP (cyclic diguanylate)
InactiveCN102443031AMeet the needs of scientific research workImprove recycling efficiencySugar derivativesSugar derivatives preparationPurification methodsFreeze-drying
The invention discloses a separation and purification method of c-di-GMP (cyclic diguanylate), comprising the following steps of: directly sampling a c-di-GMP crude product solution on a fast protein liquid chromatography equipped with an ion exchange chromatographic column; carrying out gradient elution by using a buffer solution with pH of 8.0; carrying out detection under 254 nm by an ultraviolet detector; collecting an eluent in the peak position; confirming a structure by using a mass spectrum; and freeze-drying the eluent with target compounds, thereby obtaining the solid c-di-GMP. The c-di-GMP prepared according to the method provided by the invention has the purity of over 95% through reversed-phase high performance liquid chromatography detection, and realizes c-di-GMP separation and purification from the c-di-GMP crude product solution in one step; moreover, the method provided by the invention has simple technology, short period and nonuse of toxic organic solvents, and is a novel method with high efficiency, environment friendliness and suitability for industrialized production.
Owner:SHANDONG UNIV
Detection method of carbostyril drug residue in veterinary drug
InactiveCN106908560AGuarantee drug safetyImprove securityComponent separationRetention timeProtein mass spectrometry
The invention provides a detection method of a carbostyril drug residue in a veterinary drug. The method comprises the following steps of: determining the carbostyril drug residue in the veterinary drug by means of liquid chromatography-tandem mass spectrometry; firstly, sampling a standard working liquid and a sample solution in a set liquid chromatography-tandem mass spectrometry condition; judging whether the sample contains corresponding detected objects or not, wherein the following conditions need to be met: a mass chromatographic peak retaining time in the sample solution is consistent to a mixed matrix standard working liquid with the allowable deviation of being smaller than + / -2.5%; and if the relative abundance of the set mass spectrum qualitative ions of the drug corresponding to the chromatographic peak is consistent to the relative ion abundance of the matrix standard working liquid equivalent in concentration, and the relative abundance deviation does not exceed the set specification, determining that the drug is contained. During pre-treatment of the sample by the method, the purifying process is cancelled, and the method has the characteristics of being simple in processing program, quick and high in determining accuracy rate and the like. Analyzed from a veterinary drug source, the medicinal safety of breeding industry is effectively ensured, and the animal food safety is improved.
Owner:TIANJIN SHENGJI GRP CO LTD
Matrine liquid chromatography measuring method
InactiveCN101458235AEfficient separationEffective quantificationComponent separationMatrinePhosphoric acid
The invention relates to a matrine liquid chromatographic determination method in the chemical detection technical field. The method comprises the following steps: respectively diluting a matrime sample and a matrime reference substance with methanol to volume, taking aqueous solution containing the methanol, phosphoric acid and triethylamine as a mobile phase and injecting into a liquid chromatograph, and then sequentially determining precision, 24h stability, reproducibility and recovery rate of the matrine sample. The determination method has the advantages of simple operational steps, strong adaptability and good reproducibility of analysis results.
Owner:SHANGHAI NAT ENG RES CENT FORNANOTECH
Reversed-phase liquid chromatography, liquid chromatograph apparatus, and column
InactiveUS6841073B2Reduce retention timeExtended service lifeIon-exchange process apparatusComponent separationReversed-Phase Liquid ChromatographyChromatography column
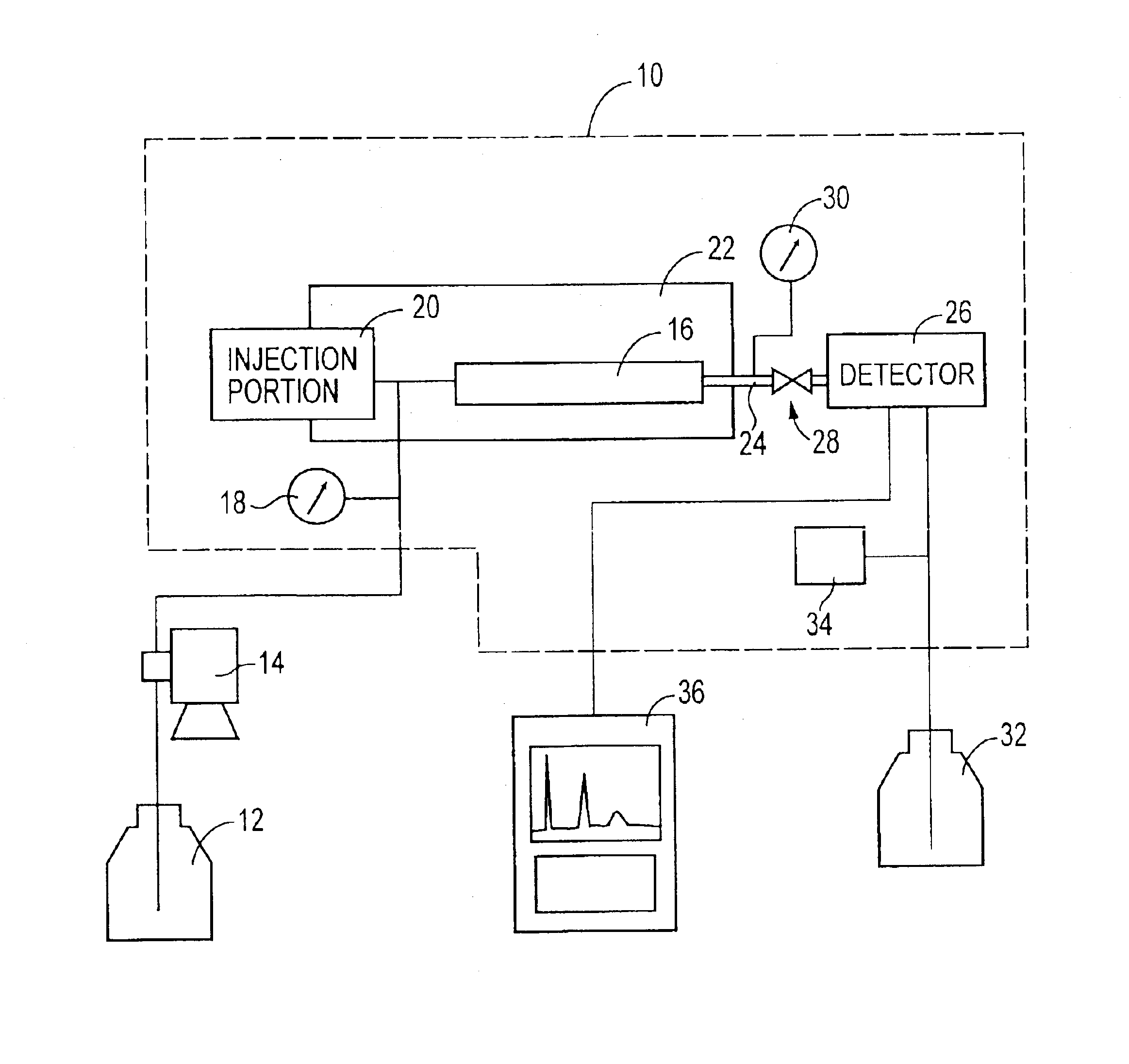
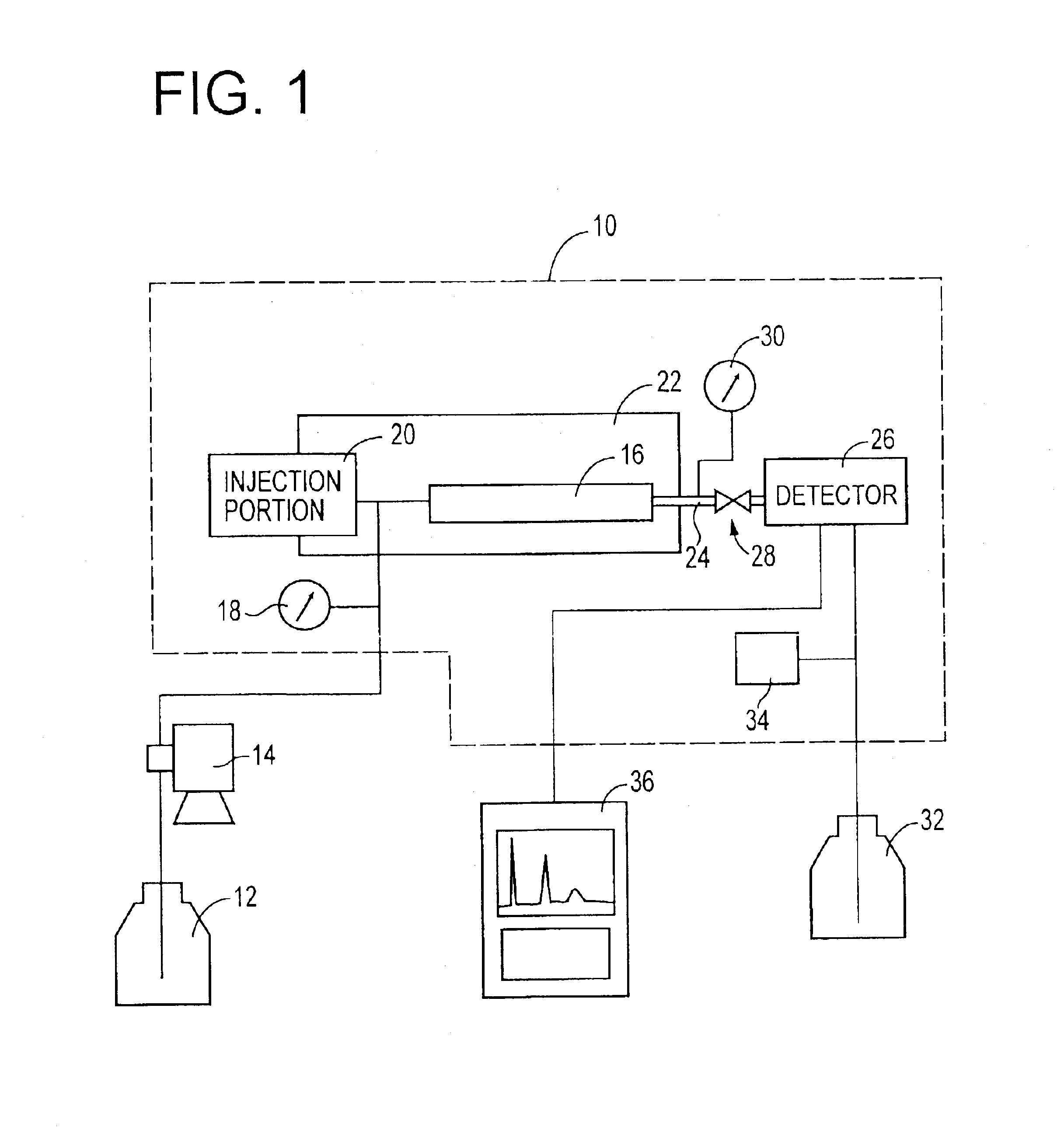
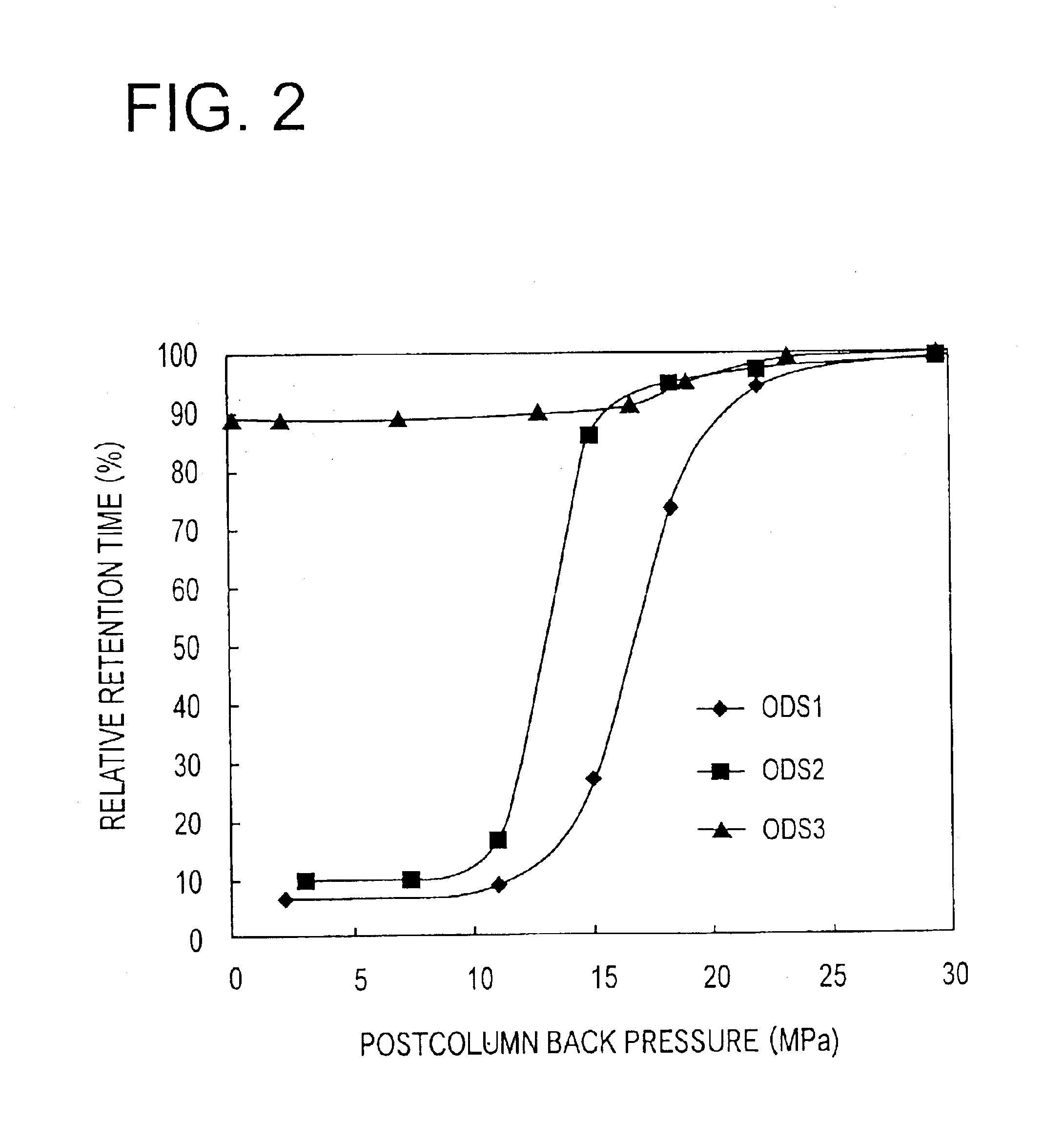
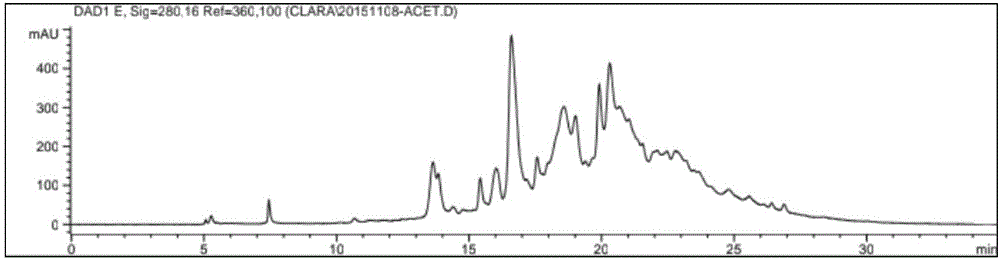
A reversed-phase liquid chromatography using a mobile phase containing water as a main component thereof, wherein in a measurement after a flow of the mobile phase through a column is temporarily stopped and then the flow is resumed, a back pressure is applied to an outlet of the column by a back-pressure applying device which is provided between the outlet of the column and a detector.
Owner:NOMURA CHEM
Preparation method for quickly separating plant proanthocyanidin dimer and trimer
The invention discloses a preparation method for quickly separating plant proanthocyanidin dimer and trimer. The method comprises the following steps: performing liquid-liquid extraction on an alcohol solution rich in plant proanthocyanidin raw material, and then flowing by a gel column, performing FPLC (Fast Protein Liquid Chromatography) or HSCCC (High Speed Counter Current Chromatography) separation, thereby separating and acquiring the proanthocyanidin dimer and trimer components. The technology disclosed by the invention is suitable for the separation and purification of the proanthocyanidin dimer and trimer from different resources and has universality. The proanthocyanidin dimer and trimer acquired according to the preparation method disclosed by the invention are higher in purity. The method has the advantages of high safety, simple operation, less time consumption, low production cost, environmental protection, reaching green chemical standard, suitability for industrial production and ultrahigh commercial value.
Owner:NANJING FORESTRY UNIV
Extraction method of human body secretory IgA, the human body secretory IgA, and O-glycosylation measurement method thereof
InactiveCN106220731AAvoid harmHigh IgA contentImmunoglobulins against animals/humansPeptide preparation methodsProtein CSerum ige
The invention belongs to the technical field of biology and provides an extraction method of human body secretory IgA, the human body secretory IgA, and an O-glycosylation measurement method thereof. The extraction method includes the steps of: firstly extracting oral cavity secretion of human body and centrifuging the oral cavity secretion; treating the oral cavity secretion to separate and purify the human body secretory immune globulin IgA by means of fast protein liquid chromatography with a Jacalin-agarose affinity chromatographic column; and performing the O-glycosylation measurement to the purified IgA. The method, compared with serum extraction, is painless, is convenient and is harmless to human body, and compared with urine IgA extraction, is high in content of the IgA after extraction and purification and is simple in separation and enrichment. The method can be widely applied in medical researching and laboratory researching.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Hindered amine light stabilizer mass control and/or identification detection method
InactiveCN104991021AFine and effective detection methodFill in the gaps in testingComponent separationGradient elutionOperability
The invention discloses a separation and analysis method based on non-aqueous reverse-phase gradient liquid chromatography. Methanol, dichloromethane and triethylamine are taken as mobile phases, a gradient elution mode is adopted, circular and linear oligomers and homologs thereof of a Chimassorb 944 product are separated for the first time, and the molecular weight of the Chimassorb 944 product is identified through MALDI-TOF; by combining with the synthetic route of the Chimassorb 944 product, it is determined that chromatographic peaks of a liquid chromatogram stand for different types of the oligomers with the molecular weights with one constitutional unit deviation and the homologs thereof, attribution is conducted on the peaks, and an effective detection method for the mass control of the Chimassorb 944 product is provided. According to the hindered amine light stabilizer mass control and / or identification detection method, instruments used in the method adopt an ordinary reverse-phase chromatographic system, the operability is strong, and important economic value is achieved by applying the method to the measurement and the product mass control of the Chimassorb 944 product.
Owner:BEIJING TIANGANG AUX CO LTD
Functionalized Support Material and Methods of Making and Using Functionalized Support Material
ActiveUS20170056854A1Cost-effectiveChromatographic cation exchangersCation exchanger materialsHigh pressureChromatography column
Methods of making functionalized support material are disclosed. Functionalized support material suitable for use in chromatography columns or cartridges, such as in a high pressure liquid chromatography (HPLC) column or a fast protein liquid chromatography (FPLC) column, is also disclosed. Chromatography columns or cartridges containing the functionalized support material, and methods of using functionalized support material, such as a media (e.g., chromatographic material) in a chromatography column or cartridge, are also disclosed.
Owner:WR GRACE & CO CONN
Method for measuring purity of papain by fast protein liquid chromatogram
InactiveCN101565739AThe assay method is fastEasy to operateComponent separationMicrobiological testing/measurementSodium acetateChromatographic separation
The invention relates to a method for measuring purity of papain by fast protein liquid chromatogram, comprising the steps of: preparing mobile phase solution A: acetic acid-sodium acetate buffer solution and mobile phase solution B: acetic acid-sodium acetate-sodium chloride buffer solution; placing refined papain and rough-wrought papain respectively into a flask with a volume of 50 ml; adding the mobile phase solution A till scale mark of 50 ml for constant volume and agitating till the papain is dissolved completely; and finally performing chromatographic separation by using an ion exchange column chromatography of the fast protein liquid chromatogram (FPLC), and calculating the purity of the papain by a formula for calculating the peak area ratio. The measuring method is fast and simple in operation, little in time consumption, reliable in result and high in repeatability, and provides basis for measuring the purity of papain precisely, thereby having important learning value andpractical application significance.
Owner:DONGHUA UNIV
Separation of glycans by mixed-mode liquid chromatography
ActiveUS9169331B2Desirable selectivityHigh resolutionOther chemical processesComponent separationChromatographic separationBinding site
An exemplary multimodal chromatographic medium of the invention includes one or more strong anion exchange, weak anion exchange, strong cation exchange and / or weak cation exchange binding sites in combination with one or more reverse phase and / or hydrophilic interaction chromatography binding site. In an exemplary embodiment, the sites interact with one or more glycans in a mixture of glycans in a manner that allows separation of glycans in the mixture and analysis of the glycan mixture. The media are incorporated into devices and systems for chromatographic analysis. Also provided are methods of using the multimodal media of the invention to analyze glycans.
Owner:DIONEX CORP
Bactrocerin, and preparation method and application thereof
InactiveCN106977589ANo hemolytic activityHas antibacterial activityAntibacterial agentsBiocideAmino acidProtein C
The invention discloses a bactrocerin, and a preparation method and an application thereof. The bactrocerin is a polypeptide separated from pupas of Bactrocera dorsalis, the molecular weight is 2.3 Dalton, the isoelectric point is 11.43, and the amino acid sequence of the bactrocerin is represented by SEQ ID NO:1. The invention also discloses the preparation method of the antimicrobial peptides of the bactrocerin. The method comprises the following steps: inducing the pupas of Bactrocera dorsalis with a mixed bacterial liquid, carrying out tissue homogenization to obtain a crude extract, and carrying out ion exchange chromatography, reversed phase fast protein liquid chromatography and reversed phase high pressure liquid chromatography purification on the crude extract to prepare the bactrocerin. The bactrocerin has an inhibition effect on Gram-positive bacteria, Gram-negative bacteria and fungi, has no hemolytic activity, can be used as a raw material for developing biotechnological products for preventing pathogenic organism infection of humans and animals, and also can be used in transgenic plants and transgenic animal breeding as an anti-disease gene through transgene and other biotechnologies.
Owner:四川科劲生物科技有限公司
A kind of plant proanthocyanidin dimer, trimer rapid separation preparation method
Owner:NANJING FORESTRY UNIV
Method of determining aspartame and alitame in food by reversed-phase liquid chromatography mixed standard sample adding method
PendingCN110632194AImprove work efficiencyImprove accuracyComponent separationAlitameReversed-Phase Liquid Chromatography
According to the method disclosed by the invention, under the same liquid chromatography condition, an aspartame and alitame mixed standard solution and the aspartame and alitame mixed standard solution added with a to-be-tested solution are injected to a liquid chromatograph respectively, qualification is carried out based on whether component signals are increased or not under the same retentiontime condition, and whether alitame or aspartame is contained in a to-be-tested food is judged; and comparison between the peak appearance signal of the mixed standard solution with the peak appearance signal difference of the mixed standard solution added with the to-be-tested solution is carried out for quantification, and the contents of the alitame and the aspartame are measured. The method is high in working efficiency, low in cost, less in interference and high in accuracy.
Owner:UNIV FOR SCI & TECH ZHENGZHOU
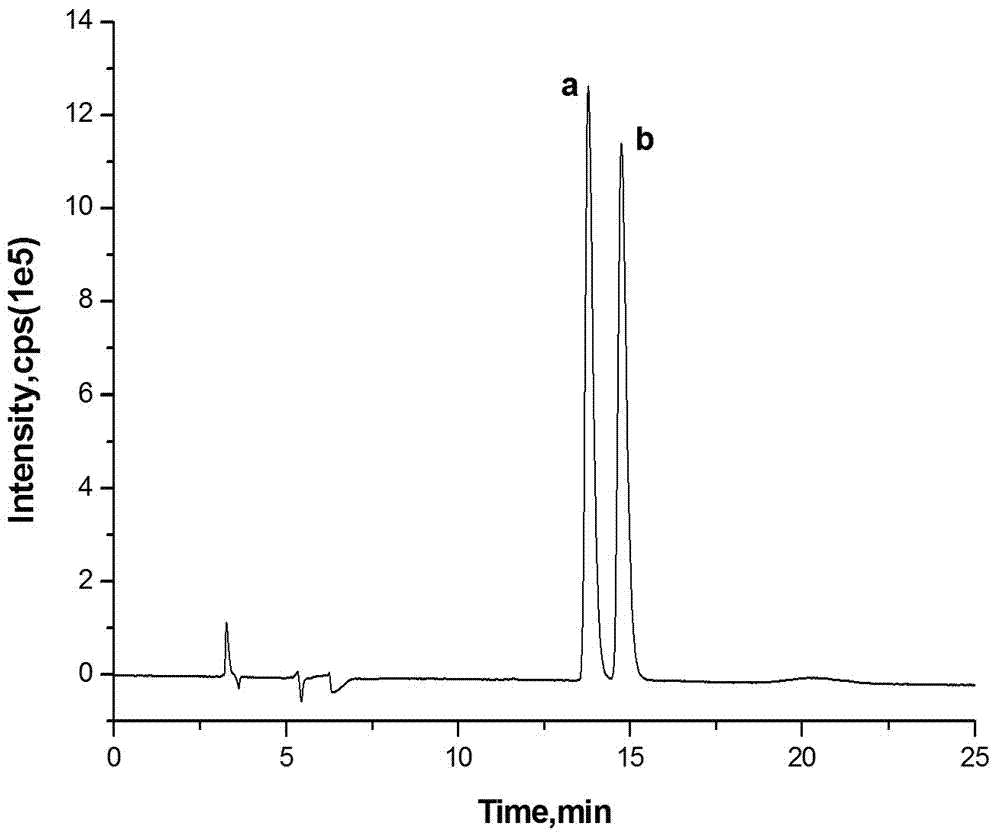
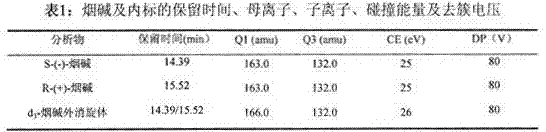
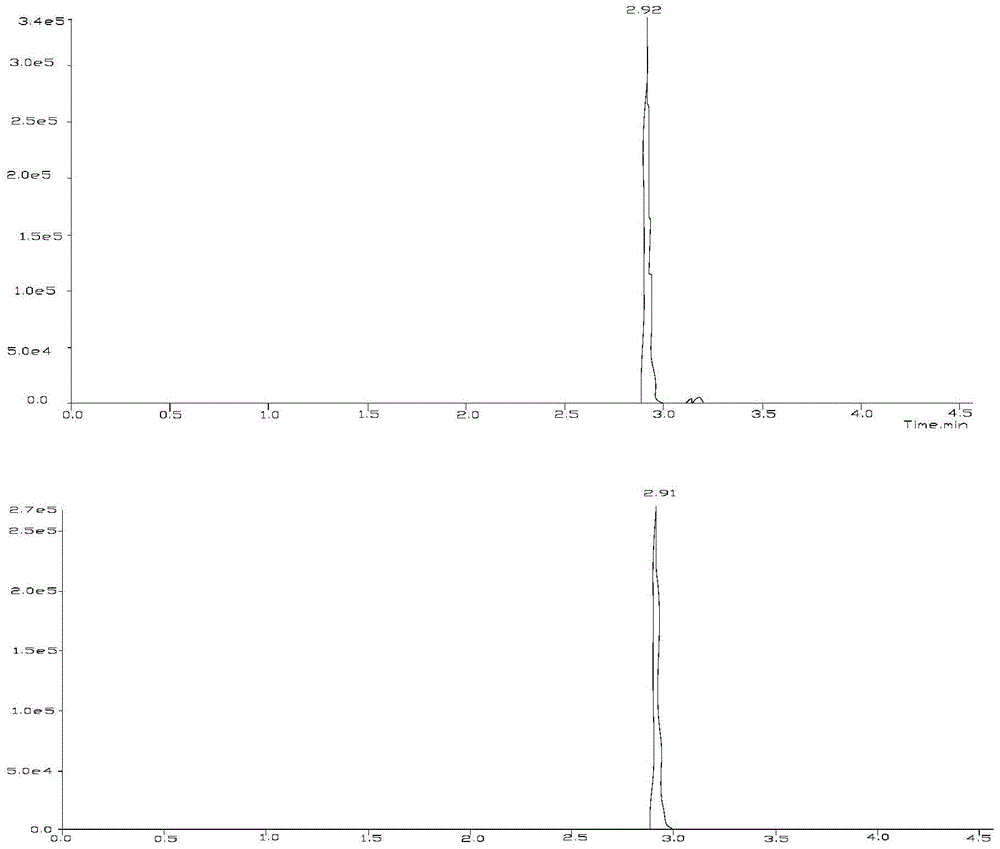
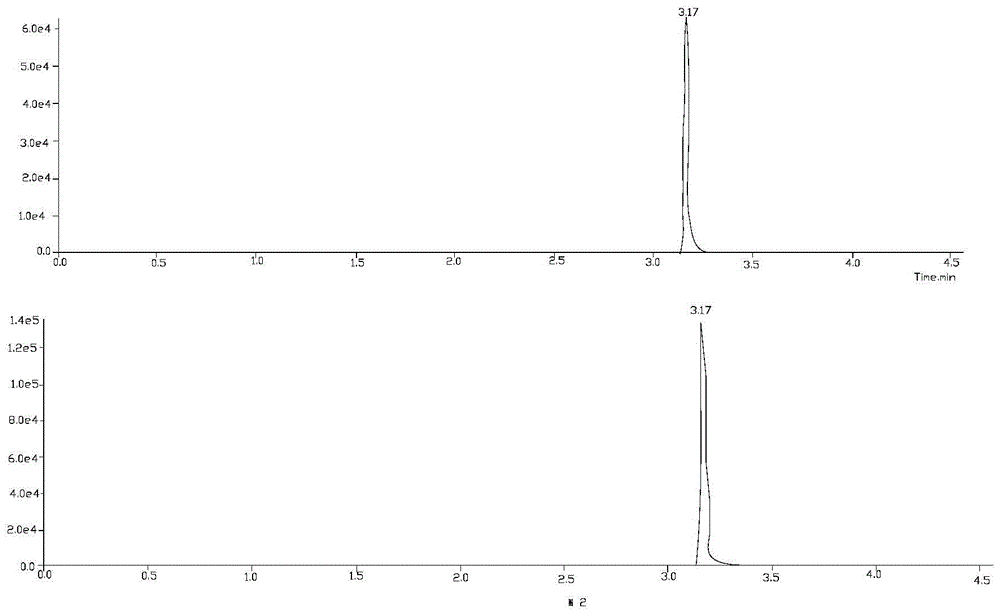
Method for analyzing nicotine optical isomers in electronic cigarette liquid by using positive-phase liquid chromatography-tandem mass spectrometry method
ActiveCN106950323AImprove ionization efficiencyHigh analytical sensitivityComponent separationChromatographic separationRetention time
The invention relates to a method for analyzing nicotine optical isomers in an electronic cigarette liquid by using a positive-phase liquid chromatography-tandem mass spectrometry method. The method is characterized in that the nicotine optical isomers in the electronic cigarette liquid are separated by a chiral chromatographic column, a post-column high pressure liquid output pump is used for introducing isopropyl alcohol and separating flow, and the tandem mass spectrometry is used for analyzing. The method has the beneficial effects that (1) the nicotine optical isomers in the electronic cigarette liquid are analyzed by the positive-phase liquid chromatography-tandem mass spectrometry method, so as to separate and detect the nicotine optical isomers in the electronic cigarette liquid; (2) the post-column high pressure liquid output pump is used for introducing the isopropyl alcohol and separating flow, so that the amount of non-polarity solvent entering mass spectrometry is reduced, the amount of the isopropyl alcohol of polarity solvent is compensated, and the ionizing efficiency is improved; (3) compared with the prior art, the chromatography resolution of the two types of nicotine optical isomers is high, and the difference of retention times is 1min; the analysis time is short, and is only 17min; the sensitivity is high, and the quantitative detection of lower content of R-(+)-nicotine in the cigarette liquid can be realized.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Liquid chromatography-tandem mass spectrometry method for triptolide A, triptolide ketone, triptolide and triptolide
The invention relates to a liquid chromatography-tandem mass spectrometry test method of wilforlide, triptonide, triptolide and tripterine. The test method includes the following steps: taking liquid samples or tissue samples, cutting into pieces and placing into a tube with a stopper; adding a certain amount of a sodium hydroxide solution into the samples to adjust the pH to be 9-10, adding ethyl acetate, oscillating for 10 minutes, and performing high speed centrifugation for 10 minutes; after separation of an organic phase, adding an organic solvent for secondary extraction, mixing the two obtained organic phases, and placing on a concentrator with the temperature of 50 DEG C for evaporation until the organic phases are dried; using an initial mobile phase to dilute the residue, enabling the obtained solution to pass through a 0.22-[um]m microporous organic membrane, and taking the filtrate for analysis by a liquid chromatography-tandem mass spectrometer. The test method provided by the invention is simple, efficient, quick, sensitive, high in accuracy and extensive in practicability, can be applied to qualitative and quantitative testing for wilforlide, triptonide, triptolide and tripterine in biological samples, and is suitable for tests on in-vitro samples and suspicious physical evidences.
Owner:INST OF FORENSIC SCI OF MIN OF PUBLIC SECURITY
Matrine liquid chromatography measuring method
The invention relates to a matrine liquid chromatographic determination method in the chemical detection technical field. The method comprises the following steps: respectively diluting a matrime sample and a matrime reference substance with methanol to volume, taking aqueous solution containing the methanol, phosphoric acid and triethylamine as a mobile phase and injecting into a liquid chromatograph, and then sequentially determining precision, 24h stability, reproducibility and recovery rate of the matrine sample. The determination method has the advantages of simple operational steps, strong adaptability and good reproducibility of analysis results.
Owner:SHANGHAI NAT ENG RES CENT FORNANOTECH
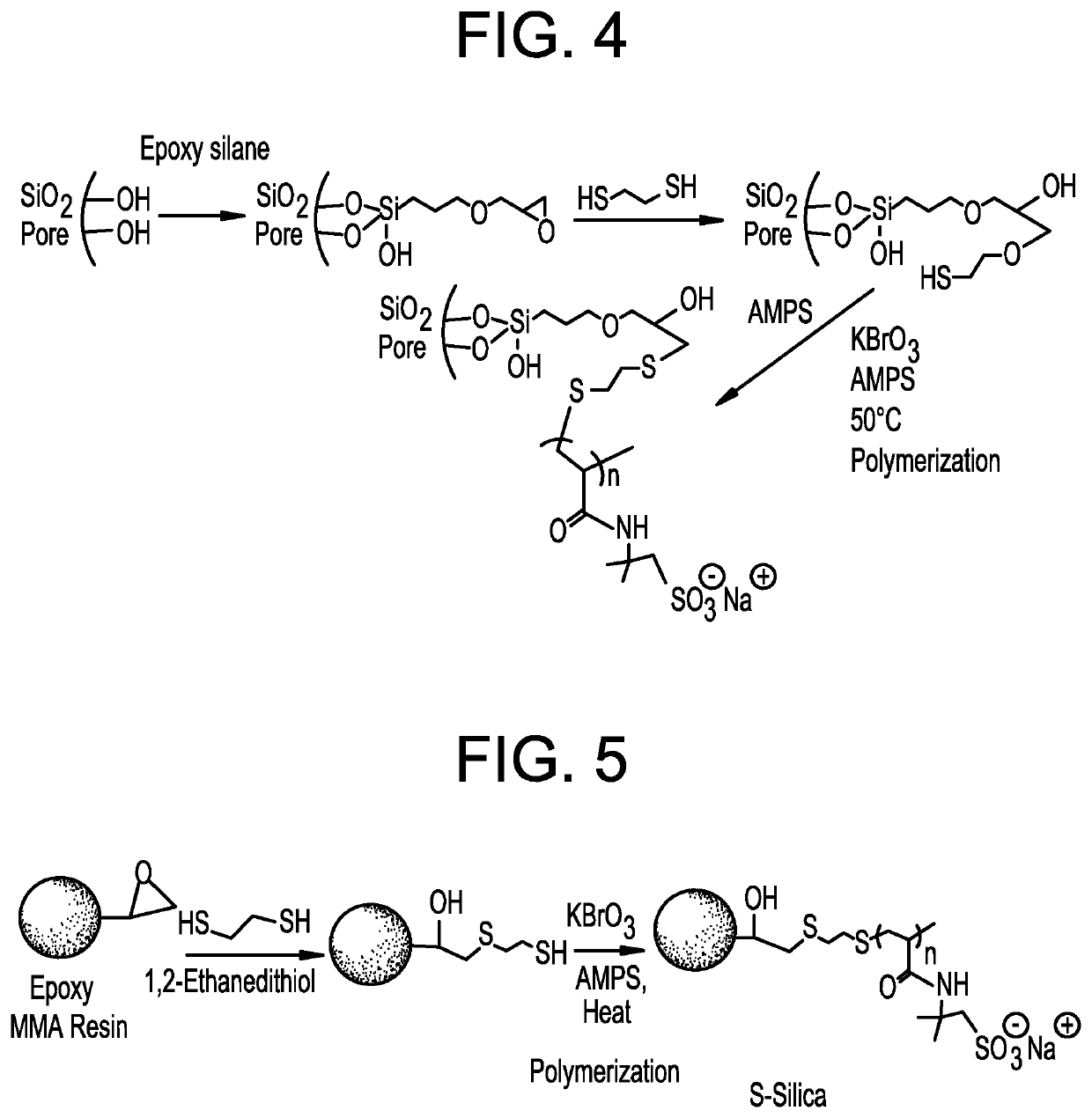
Functionalized support material and methods of making and using functionalized support material
PendingUS20220297088A1Chromatographic cation exchangersCation exchanger materialsFluid phaseOrganic chemistry
Methods of making functionalized support material am disclosed. Functionalized support material suitable for use in chromatography columns or cartridges, such as in a high pressure liquid chromatography (HPLC) column or a fast protein liquid chromatography (FPLC) column, is also disclosed. Chromatography columns or cartridges containing the functionalized support material, and methods of using functionalized support material, such as a media (e.g., chromatographic material) in a chromatography column or cartridge, are also disclosed.
Owner:WR GRACE & CO CONN
Analysis methods for various active components in oral contraceptive piacebo tablets
ActiveCN110057939ASolve the problem of requiring multiple tests to determine whether it contains the corresponding active ingredientSolve the problem of multiple tests to determine whether it contains the corresponding active ingredientComponent separationNorethisterone acetateBULK ACTIVE INGREDIENT
The invention discloses analysis methods for various active components in oral contraceptive piacebo tablets. To-be-measured piacebo tablets are placed in a measuring flask; water is added to disintegrate the tablets; an organic solvent (acetonitrile) is added and ultrasonic extraction is carried out; volume is fixed to a scale through utilization of the acetonitrile; filtering is carried out; filtrate is taken as a test sample for analysis; the test sample is analyzed through adoption of a liquid chromatography; a chromatography collection computer system is started for collection of data; norethisterone acetate, desogestrel and ethinyloestradiol reference substances are obtained; a mixed solution of the acetonitrile and the water is used for preparing a reference substance solution; andthe liquid chromatography is carried out, so whether the piacebo tablets comprise various active components or not is measured. The method has the advantages of high specificity, rapid analysis and high anti-interference performance. The problem that the various active components in the oral contraceptive piacebo tablets need to be measured repeatedly is solved.
Owner:NOVAST LABORATORIES (CHINA) LTD
Salt-tolerant liquid chromatography, electrospray mass spectrometry coupled interface device and method of using the same
ActiveCN105259273BRealize joint useSolve the problem of clogged capillaryComponent separationElectro sprayEngineering
The invention provides an anti-salt liquid chromatogram and electro-mist mass spectrometry combined interface device. The device mainly comprises a liquid phase mist generation module, a rotary barrier piece module, a probe high-voltage make-and-break module and a power module, the rotary barrier piece module comprises an insulating part and a barrier piece, one end of the insulating part is fixedly connected with the barrier piece, and the barrier piece is composed of barrier blades and barrier piece notches; one end of the probe high-voltage make-and-break module is connected with high voltage, and the other end of the probe high-voltage make-and-break module is connected with a conducting probe. According to the anti-salt liquid chromatogram electro-spray mass spectrometry combined interface device, by selecting the barrier piece notches of different sector angles or adjusting the rotation speed of the power module, the sampling capacity (the sampling quantity is generally several nano-liters) of the PESI conducting probe is satisfied, and combination of the liquid phase and the mass spectrum (PESI) is achieved. Meanwhile, the sharp end of the conducting probe generates electro-mist, so that unionized salt is deposited on the surface of the conducting probe, the problem that a high-salt sample access is blocked due to salt deposition is avoided, the detection sensitivity of the mass spectrum for a target compound in a high-salt base material is enhanced, and the liquid chromatogram and mass spectrometry coupling technology of a high-salt sample is achieved.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Method for Determination of Tatb Synthesis Intermediates and Products in Wastewater by Liquid Chromatography
ActiveCN104237405BAccurate detectionReduce detection errorComponent separationSynthesis methodsMicrofiltration membrane
The invention relates to a method for determining synthesized intermediate and product in wastewater in TATB production by liquid chromatogram, belonging to the technical field of chemical analysis. The method comprises the following steps: firstly, preparing standard solutions of phloroglucinol, TETNB and TATB standard substances by using liquid phase chromatogram mobile phase; secondly, treating the TATB wastewater, regulating pH to be neutral, extracting to-be-detected substance, drying the to-be-detected substance, adding the liquid phase chromatogram mobile phase for dissolving, filtering through a microfiltration membrane to remove impurity to obtain a liquid phase chromatogram detection sample; thirdly, performing liquid phase chromatographic analysis on the standard solutions, and drawing a standard curve; and fourthly, respectively analyzing the samples by using the liquid phase chromatogram, comparing the analysis result with the standard curve to determine the intermediate and the product in the TATB wastewater. Trace amount of intermediate related substances, such as phloroglucinol, TETNB and the product, namely TATB in the TATB wastewater can be accurately detected by using the method disclosed by the invention, and the defects in the prior art are filled.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Separation and purification method of c-di-GMP (cyclic diguanylate)
InactiveCN102443031BMeet the needs of scientific research workImprove recycling efficiencySugar derivativesSugar derivatives preparationPurification methodsFreeze-drying
The invention discloses a separation and purification method of c-di-GMP (cyclic diguanylate), comprising the following steps of: directly sampling a c-di-GMP crude product solution on a fast protein liquid chromatography equipped with an ion exchange chromatographic column; carrying out gradient elution by using a buffer solution with pH of 8.0; carrying out detection under 254 nm by an ultraviolet detector; collecting an eluent in the peak position; confirming a structure by using a mass spectrum; and freeze-drying the eluent with target compounds, thereby obtaining the solid c-di-GMP. The c-di-GMP prepared according to the method provided by the invention has the purity of over 95% through reversed-phase high performance liquid chromatography detection, and realizes c-di-GMP separation and purification from the c-di-GMP crude product solution in one step; moreover, the method provided by the invention has simple technology, short period and nonuse of toxic organic solvents, and is a novel method with high efficiency, environment friendliness and suitability for industrialized production.
Owner:SHANDONG UNIV
Functionalized support material and methods of making and using functionalized support material
ActiveUS11389783B2Chromatographic cation exchangersCation exchanger materialsFluid phaseOrganic chemistry
Methods of making functionalized support material are disclosed. Functionalized support material suitable for use in chromatography columns or cartridges, such as in a high pressure liquid chromatography (HPLC) column or a fast protein liquid chromatography (FPLC) column, is also disclosed. Chromatography columns or cartridges containing the functionalized support material, and methods of using functionalized support material, such as a media (e.g., chromatographic material) in a chromatography column or cartridge, are also disclosed.
Owner:WR GRACE & CO
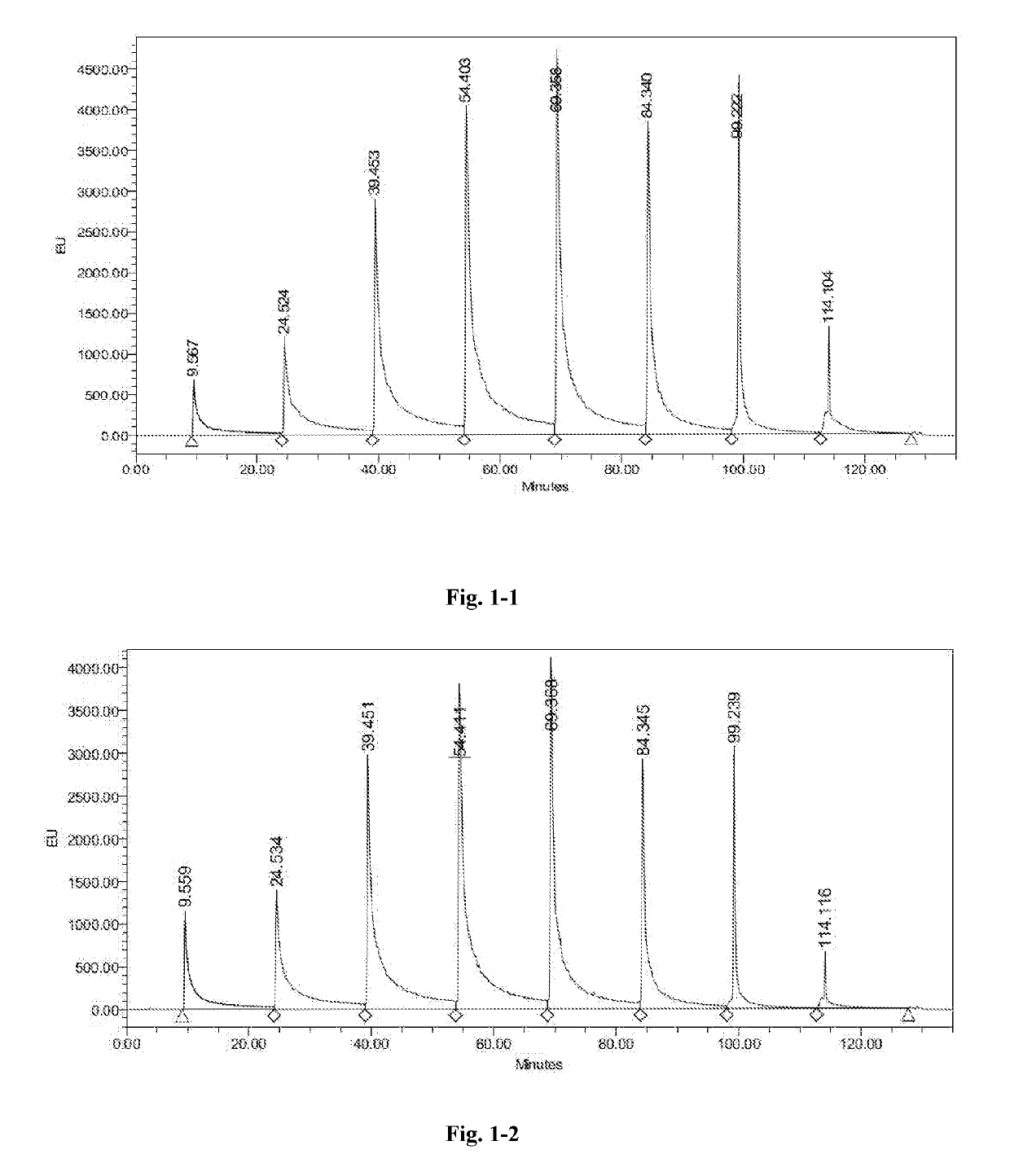
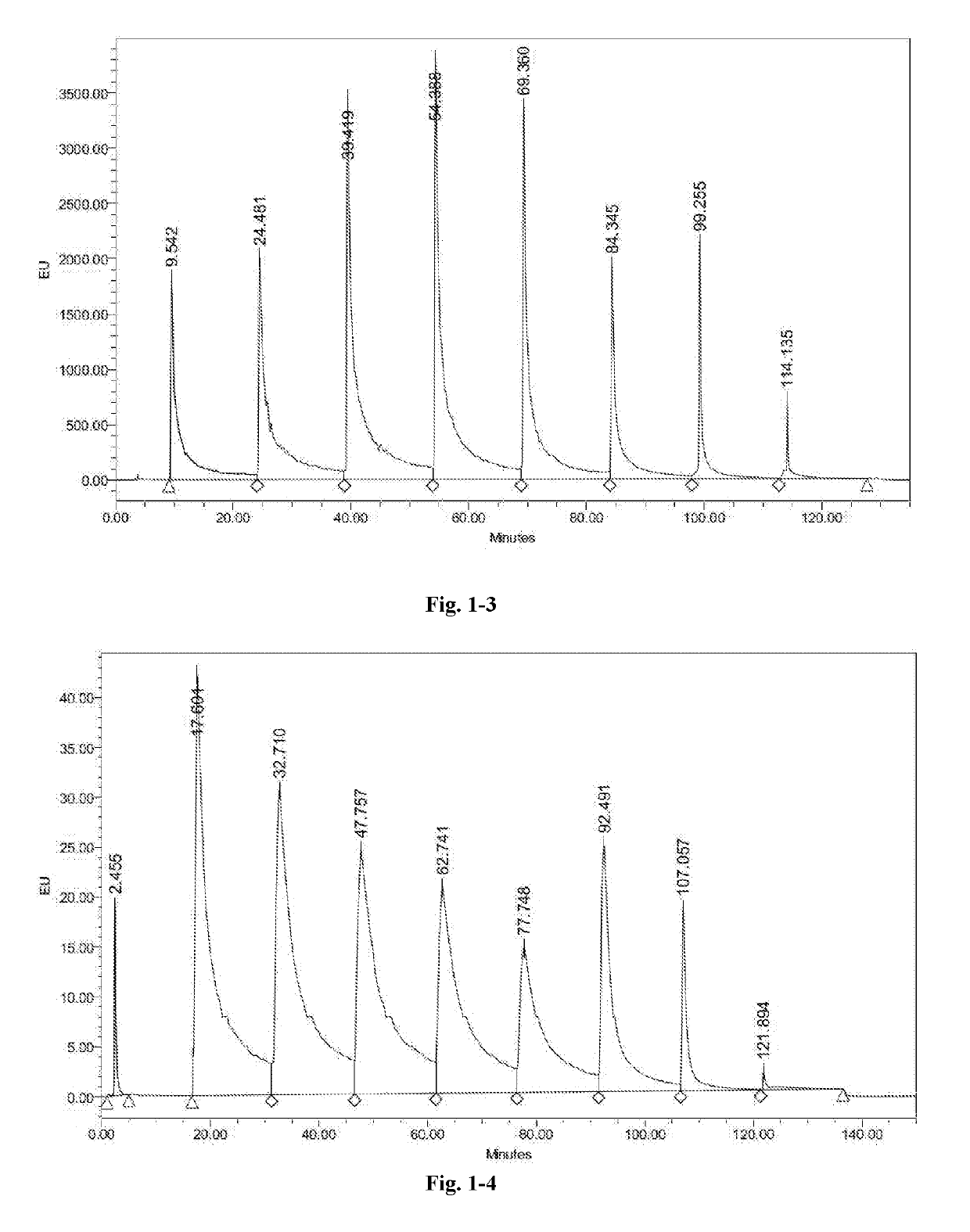
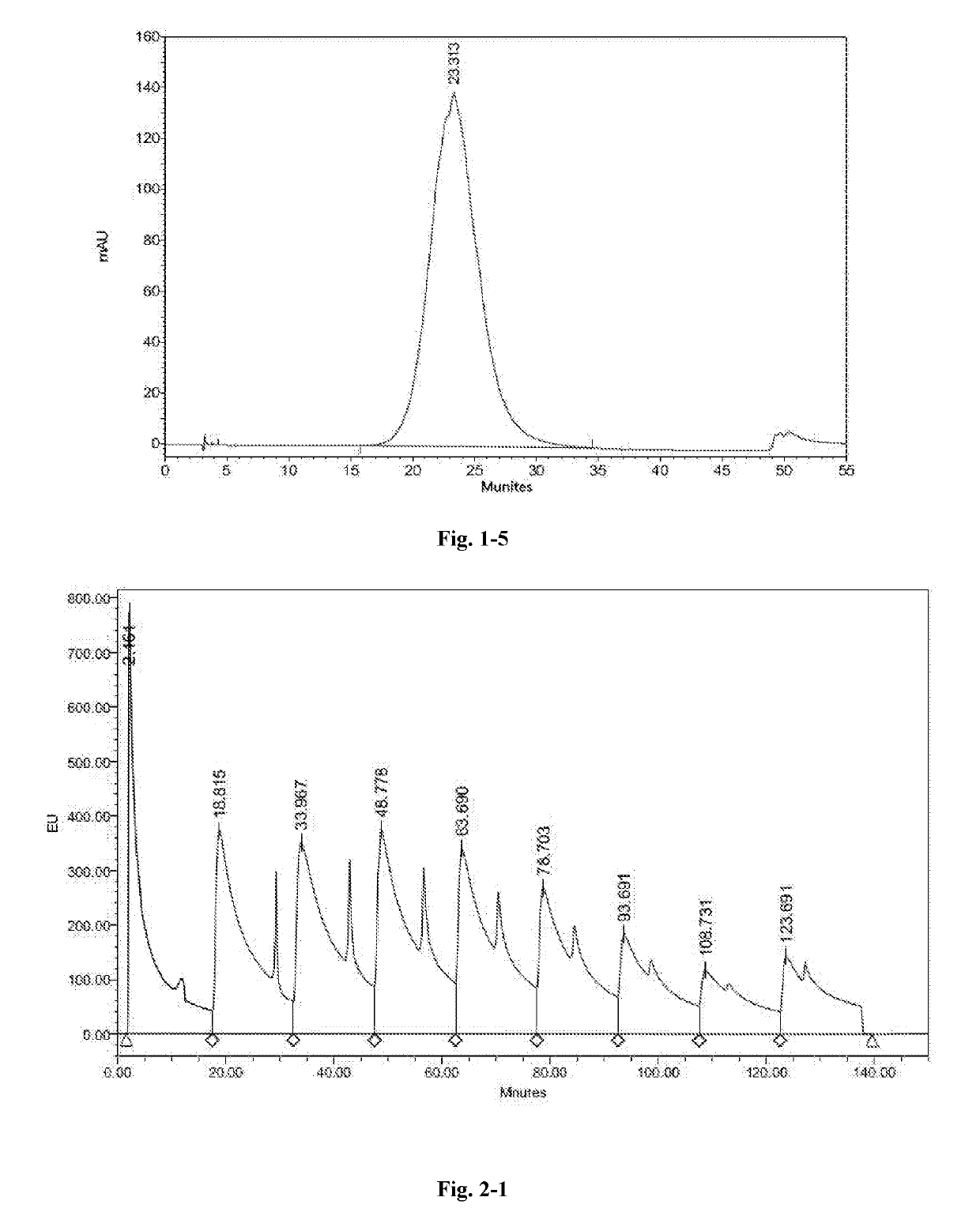
High performance liquid chromatography method for polypeptide mixtures
ActiveUS10330650B2Nervous disorderCation exchanger materialsReversed-Phase Liquid ChromatographyIon exchange
The present invention relates to a high performance liquid chromatography method for polypeptide mixtures. Specifically, the method including the following steps: step (1): preparing a solution of the glatiramer acetate to be tested; step (2): performing gradient elution on a sample to be tested with an anion exchange liquid chromatography, a cation exchange liquid chromatography, or a reversed-phase liquid chromatography; step (3): determining a peak area corresponding to each component of the glatiramer acetate, comparing the peak area with to a peak area of a reference substance to determine whether the content of each component of the sample to be tested is in a qualified range.
Owner:HYBIO PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com