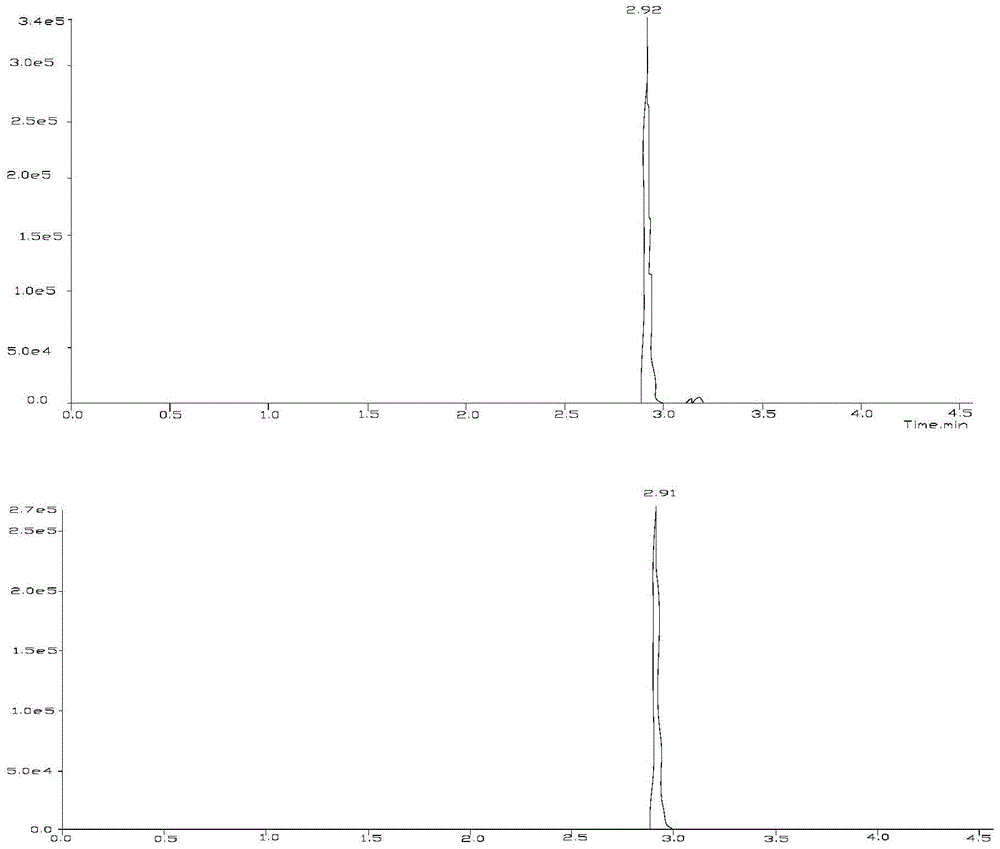
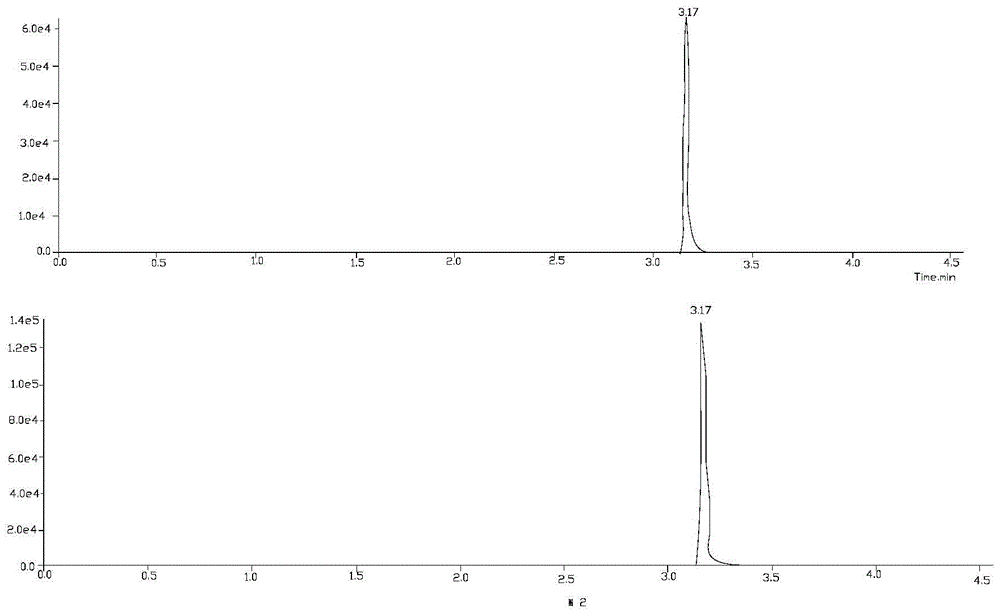
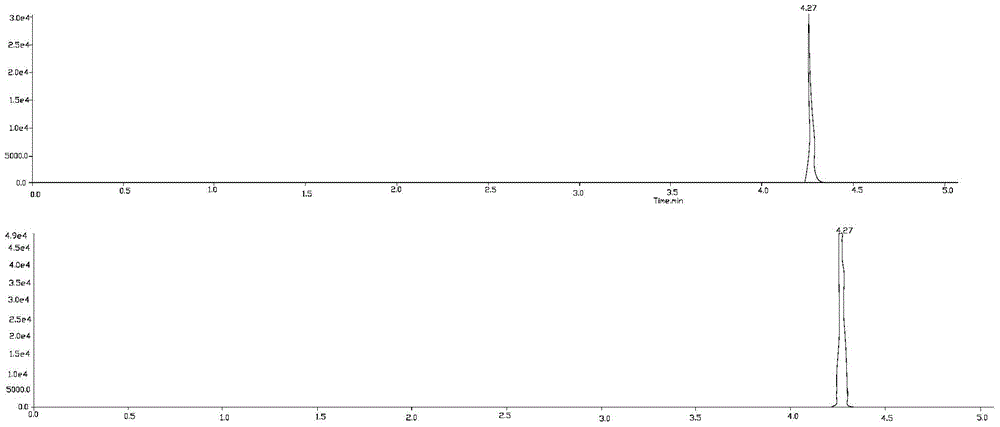
Liquid chromatography-tandem mass spectrometry method for triptolide A, triptolide ketone, triptolide and triptolide
A technology of triptolide ketone and triptolide A, which is applied in the field of biological sample inspection, can solve the problem of high weight content, and achieve the effects of high quantitative accuracy, simple operation, and less sample consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 qualitative analysis
[0027] 1. Reagents
[0028] The test water of the present invention is primary water (see GB / T 6682-2008 regulations):
[0029] a) Sodium dihydrogen phosphate, disodium hydrogen phosphate, and sodium hydroxide are analytically pure;
[0030] b) Methanol, acetonitrile, diethylamine, isopropanol, ammonium acetate, formic acid, ethyl acetate, and cyclohexane are all chromatographically pure;
[0031] c) Phosphate buffer solution (pH 5.8): Take 8.34 g of potassium dihydrogen phosphate and 0.87 g of dipotassium hydrogen phosphate, put them in a 1000 mL volumetric flask respectively, add grade 1 water to dissolve and dilute to the mark;
[0032] d) 10mM ammonium acetate containing 0.1% formic acid: weigh 0.384g of ammonium acetate, add a certain amount of first-grade water to dissolve, add 0.5mL formic acid and mix well, and dilute the mixture to 500mL with first-grade water;
[0033] e) Standard solution:
[0034] 1) Triptolide A, trip...
Embodiment 2
[0094] Embodiment 2 quantitative analysis
[0095] 1, reagent is the same as embodiment 1
[0096] 2, instrument and material are the same as embodiment 1
[0097] 3. Sample extraction
[0098] Accurately measure 1.0mL-2.0mL or 1.0g-2.0g of the case sample in parallel; take two blank samples of the same matrix, and add the corresponding target standard (the amount added should be similar to the rough measurement of the case sample) to prepare Add sample, mix well. Others are the same as embodiment 1.
[0099] 4. Instrument detection is the same as in Example 1
[0100] 5. Record and calculate
[0101] 5.1. Calculate drug content
[0102] Record the peak area value of the target substance in 2 to 3 parallel injections of each sample, and calculate the content according to the formula (2):
[0103]
[0104] In the formula:
[0105] W - the content of the target substance in the sample per unit mass, in micrograms per gram (μg / g) or micrograms per milliliter (μg / mL); ...
Embodiment 3
[0121] Embodiment 3 utilizes non-fresh blood to evaluate the method of the present invention
[0122] 1, reagent, with embodiment 1.
[0123] 2, apparatus and material, with embodiment 1.
[0124] 3. Sample extraction
[0125] Take 2.0 mL of blank non-fresh blood in a stoppered test tube, add triptolide A, triptolide ketone, triptolide and triptolide standard products respectively to prepare added samples, and mix well.
[0126] A certain amount of sodium hydroxide solution was added to the above samples to adjust the pH to 9, 10.0 mL of ethyl acetate was added, shaken for 10 min, and centrifuged at high speed for 10 min. After separating the organic phase, add an organic solvent to carry out the second extraction, combine two organic phases (the inorganic phase after the second extraction is carried out to high-performance liquid chromatography analysis, the extraction rate of the sample is 99.9%), and place it in a 50°C concentrator Wipe dry. The residue was fixed to vol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com