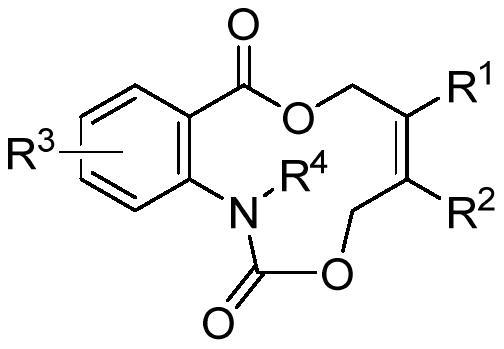
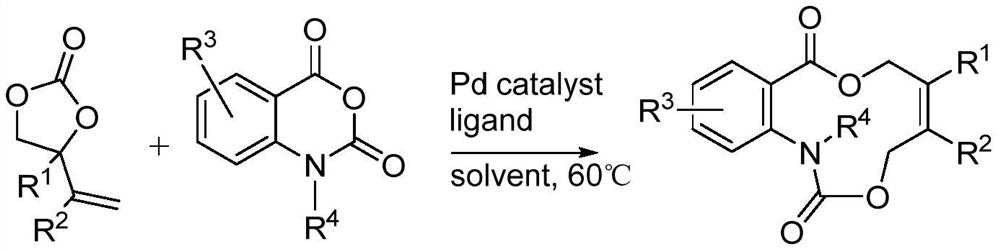
Aza medium ring lactone compound and preparation method thereof
A technology for cyclic lactones and compounds, applied in the field of compound preparation, can solve rare problems and the like, and achieve the effects of short reaction time, high product yield, and convenient and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
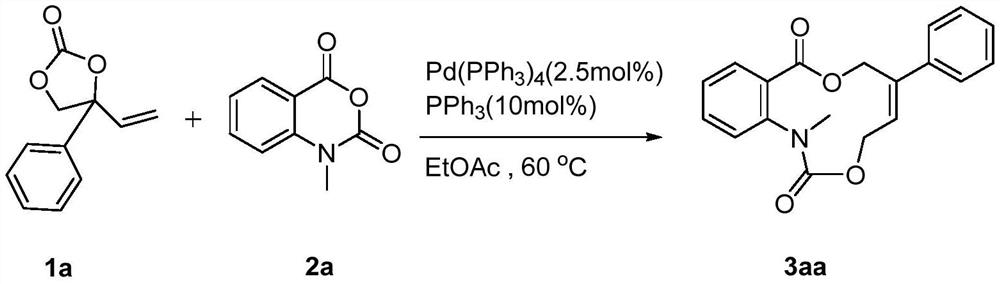
[0034] Weigh 1a (38.0mg, 0.2mmol), 2a (17.7mg, 0.1mmol), palladium catalyst (2.9mg, 0.0025mmol) and ligand PPh 3 (2.6mg, 0.01mmol) was dissolved in 1mL of ethyl acetate, heated and stirred at 60°C for 5 hours (reaction detected by TLC), after the reaction was complete, the crude product was subjected to column chromatography to obtain the target product 3aa (31.8mg) , the yield was 98%.
[0035] Characterization and analysis of target objects:
[0036] 3aa: white solid, 1 H NMR (400MHz, CDCl 3 ): δ7.58(d, J=7.2Hz, 1H), 7.51-7.47(m, 3H), 7.41-7.33(m, 3H), 7.27-7.25(m, 2H), 6.11(t, J=6.8 Hz, 1H), 5.26(s, 2H), 4.69(d, J=6.0Hz, 2H), 3.45(s, 3H)ppm; 13 C NMR (100MHz, CDCl 3 ): δ167.9, 154.2, 145.8, 141.1, 140.8, 131.3, 130.0, 128.6, 128.2, 127.5, 126.8, 125.4, 124.6, 124.5, 64.1, 61.6, 38.1ppm; HRMS (ESI) m / z: C 19 h 17 NO 4 [M+H] + The theoretical calculation value is 324.1230, and the measured value is 324.1218.
Embodiment 2
[0038]
[0039] Weigh 1a (38.0mg, 0.2mmol), 2b (21.2mg, 0.1mmol), palladium catalyst (2.9mg, 0.0025mmol) and ligand PPh 3 (2.6mg, 0.01mmol) was dissolved in 1mL of ethyl acetate, heated and stirred at 60°C for 4 hours (reaction detected by TLC), after the reaction was complete, the crude product was subjected to column chromatography to obtain the target product 3ab (31.1mg) , the yield was 87%.
[0040] Characterization and analysis of target objects:
[0041] 3ab: white solid, 1 H NMR (400MHz, CDCl 3 ): δ7.55(s,1H),7.49(d,6.4Hz,2H),7.45-7.36(m,4H),7.20(d,J=8.8Hz,1H),6.13(t,J=8.0Hz ,1H),5.26(s,2H),4.68(d,J=4.8Hz,2H),3.42(s,3H)ppm; 13 C NMR (100MHz, CDCl 3 ): δ166.6, 153.9, 145.9, 140.8, 139.4, 131.4, 131.2, 131.0, 128.6, 128.3, 127.5, 126.8, 125.9, 124.4, 64.3, 61.6, 38.1ppm; HRMS (ESI) m / z: C 19 h 16 ClNO 4 [M+H] + The theoretically calculated value is 358.0841, and the measured value is 358.0827.
Embodiment 3
[0043]
[0044] Weigh 1b (53.8mg, 0.2mmol), 2a (17.7mg, 0.1mmol), palladium catalyst (2.9mg, 0.0025mmol) and ligand PPh 3(2.6mg, 0.01mmol) was dissolved in 1mL of ethyl acetate, heated and stirred at 60°C for 2 hours (reaction detected by TLC), after the reaction was complete, the crude product was subjected to column chromatography to obtain the target product 3ba (34.9mg) , the yield was 87%.
[0045] Characterization and analysis of target objects:
[0046] 3ba: white solid, 1 H NMR (400MHz, CDCl 3 ): δ7.57(d, J=7.2Hz, 1H), 7.51-7.47(m, 3H), 7.36(d, J=8.0Hz, 2H), 7.27-7.25(m, 2H), 6.09(t, J=6.4Hz, 1H), 5.19(s, 2H), 4.67(d, J=4.8Hz, 2H), 3.43(s, 3H)ppm; 13 C NMR (100MHz, CDCl 3 ): δ167.8, 154.1, 144.5, 140.9, 140.1, 131.7, 131.3, 130.0, 128.5, 127.5, 125.5, 125.0, 124.6, 122.3, 64.1, 61.3, 38.1ppm; HRMS (ESI) m / z: C 19 h 16 BrNO 4 [M+H] + The theoretical calculation value is 402.0336, and the measured value is 402.0321.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com